* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Nuclear Fission sim
Molecular Hamiltonian wikipedia , lookup
Technetium-99m wikipedia , lookup
Nuclear fusion wikipedia , lookup
Rutherford backscattering spectrometry wikipedia , lookup
Nuclear binding energy wikipedia , lookup
Nuclear and radiation accidents and incidents wikipedia , lookup
Nuclear fission wikipedia , lookup
Nuclear fission product wikipedia , lookup
Nuclear fusion–fission hybrid wikipedia , lookup
Atomic theory wikipedia , lookup
Atomic nucleus wikipedia , lookup
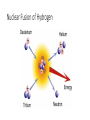
Nuclear Chemistry Vocabulary: copy the definitions • Radiation—the emission of particles or electromagnetic waves (such as visible light, X-rays, and microwaves). • Nuclear radiation—a form of ionizing radiation that is caused by changes in the nuclei of atoms. • Radioactivity—the spontaneous emission of particles and energy from atomic nuclei. Electromagnetic spectrum Radioactive Decay • In chemical reactions, the atomic number does not change; an atom of aluminum (13 protons) always remains an aluminum atom. • However, atoms with unstable nuclei—radioactive atoms— can spontaneously change their identities. When this happens, the identity of the radioactive atom usually changes because the number of protons changes, and it becomes an atom of a different element. This process is radioactive decay. Alpha (α) Decay: Copy for notes The nucleus of the parent atom decays by emitting an alpha particle (a helium nucleus with two protons and two neutrons). The daughter atom’s atomic number decreases by two; its mass number decreases by four. Beta (β) Decay: Copy for notes In beta decay, a neutron (either by itself or inside a nucleus) spontaneously changes into a proton by emitting a high-energy electron (the β particle, up to 0.9c). Notice the atomic increases by one but the mass stays the same. Carbon-14 (atomic number is 6) to Nitrogen (atomic number is 7). Gamma (γ) Decay: Copy for notes Gamma decay often follows almost immediately after α and β decay because the daughter nucleus is in an energetically excited state that is unstable so it releases a high-energy photon called a gamma ray to achieve stability. Penetration of Ionizing Radiation Which is the most damaging? • In general the greater the charge and mass of the type of radiation, the more damage it does when it hits or collides, but this also means it travels less distance through air or other matter before giving up its energy. • α particles do the most damage because they have the greatest charge and mass and easily ionize any atom they come near but they are the easiest to shield against. • β particles do less damage than α particles, but they are harder to protect against. • γ rays cause the least amount of ionization, but they are the hardest to stop. Radon Gas • Radon is the heaviest noble gas and naturally occurs in Earth’s atmosphere as a decay product of Uranium238 in soils and building materials. • It has become a health issue as homes have become more airtight as we strive to conserve energy. Remedies include sealing cracks in floors and increasing ventilation. • When radon is inhaled, it decays to produce heavy metal ions of polonium, bismuth and lead which cannot be exhaled so they stay in the body and emit damaging alpha particles. Half-Lives of Various Isotopes Radioactive Isotope Half-Life Hydrogen-3 12.3 years Carbon-14 5730 years Radon-222 3.82 days Potassium-40 1.28 billion years Oxygen-15 122.22 seconds Uranium-235 703 million years Plutonium-239 24,100 years Why do we care about half-lives? Knowing how long a radioisotope used in a medical test will remain active in the body lets doctors get information but minimize harm to a patient. We can plan how to best store hazardous nuclear waste from nuclear power plants. Scientists can estimate the ages of bones, metal objects, or rocks. Nuclear Fission: copy for notes • Splitting the nucleus of an atom into two smaller nuclei is nuclear fission. • The nuclear fission of heavy atoms such as uranium releases huge amounts of energy, much more (a million times!) than is released in a chemical reaction. • This energy comes from converting the small amount of mass that is lost during fission into energy. (E=mc2, you know!) Fission of Uranium-235: Copy for notes Nuclear Fission sim Nuclear Fusion: Copy for notes • Nuclear fusion is the combining or fusing of two smaller nuclei into the nucleus of a larger atom. • This process occurs inside of stars and powers our sun, which burns hydrogen gas and creates helium and heavier elements. • Once again, the creation of new elements results in a small mass difference, and this mass is converted into energy which the sun radiates into space and gives us heat and light. Nuclear Fusion of Hydrogen Why do fission and fusion produce so much energy? • Nuclear fission and fusion are the splitting and combining of NUCLEI and their bonds, while chemical reactions involve the bonds of atoms’ ELECTRONS. • The energy in chemical reactions comes from the difference between electrons’ bonds in reactants and in products. Energy is released as heat, when the bonds of products are stronger than in reactants. • Both mass and energy are conserved in chemical reactions. Why do fission and fusion produce so much energy? • In nuclear reactions, energy comes from converting tiny amounts of mass lost when the bonds between protons and neutrons are broken and made. These bonds are due to the strong force. • The strong force is a thousand times stronger than the electrical force which holds atoms and ions together in chemical bonds. • However, the energy produced by the fission or fusion of just one atom is too small to be practical. Chain Reaction • In order for nuclear reactions to produce practical amounts of energy, there have to be a LOT of them. How can we do that? • Some fissionable materials have the property that, while they require one neutron to fission, they produce more than one neutron. This means that, if you have enough atoms (a CRITICAL MASS), the one neutron you started with quickly produces more neutrons which cause more and more atoms to fission, producing even more neutrons. The reaction continues until you run out of fissionable material. This is called a CHAIN REACTION. Fission of Uranium-235 Watch the “Nuclear Fission” sim Nuclear Power Plants • Instead of using fossil fuel combustion to boil water to produce steam to turn turbines to generate electricity, nuclear power plants use heat released from nuclear fission reactions to boil the water. • The essential parts of a nuclear power plant are the fuel rods, control rods, moderator, generator, and cooling system. New Mexico’s Role in Nuclear Science Los Alamos Research Center, 1943 - present Founded during World War II as a secret, centralized facility for scientific research on the Manhattan Project, the project to develop the first nuclear weapons. After the Cold War, research emphasis has shifted to include medicine (vaccines for AIDS, breast cancer) and renewable energy. White Sands, Alamogordo, NM, July, 1945 First test of a nuclear weapon, ‘Trinity’ was a plutoniumimplosion device (20 kilotons TNT) detonated above ground. Considered to be the start of the ‘Atomic Age.’ New Mexico’s Role in Nuclear Science Sandia National Labs, Kirtland AFB near Albuquerque, 1949-present Sandia Labs’ “mission [is] to maintain the reliability and surety of nuclear weapon systems, conduct research and development in arms control and nonproliferation technologies, and investigate methods for the disposal of the United States' nuclear weapons program's hazardous waste.” (Wikipedia, 4/17/13) Other research focuses on energy, the environment, critical national infrastructures, computational biology, mathematics, materials science, alternative energy, and cognitive science. New Mexico’s Role in Nuclear Science Waste Isolation Pilot Plant (WIPP), near Carlsbad, 1971 present • Repository for transuranic waste including materials such as gloves, tools, rags, and assorted machinery which have come in contact with radioactive substances (mostly plutonium and uranium) in the production of nuclear fuel and weapons. • Designed to store this waste in rooms in stable salt formations for 10,000 years. • First waste arrived in 1999; will accept waste for another 25-35 years then be sealed. New Mexico’s Role in Nuclear Science National Enrichment Facility, URENCO, Eunice, 2006present (began enriching 2010) Plant for the enrichment of uranium for use in nuclear power plants. Natural uranium has only 0.72% U-235 (the fissionable isotope), so this plant processes it into low-enriched, reactor grade uranium (3-4% U-235). Waste Control Specialists, Andrews, TX, 2009 – present (actually 5 mi east of Eunice) Treatment, storage, & disposal company dealing in low-level radioactive, hazardous, and mixed wastes.