* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Feb20_modified
Mössbauer spectroscopy wikipedia , lookup
Anti-reflective coating wikipedia , lookup
Atmospheric optics wikipedia , lookup
Retroreflector wikipedia , lookup
Photomultiplier wikipedia , lookup
Rutherford backscattering spectrometry wikipedia , lookup
Thomas Young (scientist) wikipedia , lookup
Nonlinear optics wikipedia , lookup
Upconverting nanoparticles wikipedia , lookup
Ultrafast laser spectroscopy wikipedia , lookup
Magnetic circular dichroism wikipedia , lookup
Population inversion wikipedia , lookup
Ultraviolet–visible spectroscopy wikipedia , lookup
Transparency and translucency wikipedia , lookup
Astronomical spectroscopy wikipedia , lookup
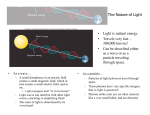
Homework 4 Unit 21 Problem 17, 18, 19 Unit 23 Problem 9, 10, 13, 15, 17, 18, 19, 20 Units 21 and 22 are covered The Nature of Light • Light is radiant energy. • Travels very fast – 300,000 km/sec! • Can be described either as a wave or as a particle traveling through space. • • As a wave… – A small disturbance in an electric field creates a small magnetic field, which in turn creates a small electric field, and so on… • Light propagates itself “by its bootstraps!” – Light waves can interfere with other light waves, canceling or amplifying them! – The color of light is determined by its wavelength. As a particle… – Particles of light (photons) travel through space. – These photons have very specific energies. that is, light is quantized. – Photons strike your eye (or other sensors) like a very small bullet, and are detected. The Effect of Distance on Light • Light from distant objects seems very dim – Why? Is it because the photons are losing energy? – No – the light is simply spreading out as it travels from its source to its destination – The farther from the source you are, the dimmer the light seems – We say that the object’s brightness, or amount of light received from a source, is decreasing Brightness = Total Light Output 4pd 2 This is an inverse-square law – the brightness decreases as the square of the distance (d) from the source The Nature of Matter • The atom has a nucleus at its center containing protons and neutrons • Outside of the nucleus, electrons whiz around in clouds called orbitals – Electrons can also be described using wave or particle models – Electron orbitals are quantized – that is, they exist only at very particular energies • – The lowest energy orbital is called the ground state, one electron wave long • To move an electron from one orbital to the next higher one, a specific amount of energy must be added. Likewise, a specific amount of energy must be released for an electron to move to a lower orbital These are called electronic transitions Measuring Temperature • It is useful to think of temperature in a slightly different way than we are accustomed to – Temperature is a measure of the motion of atoms in an object – Objects with low temperatures have atoms that are not moving much – Objects with high temperatures have atoms that are moving around very rapidly • The Kelvin temperature scale was designed to reflect this – 0 K is absolute zero –the atoms in an object are not moving at all! Results of More Collisions • Additional collisions mean that more photons are emitted, so the object gets brighter • Additional hard collisions means that more photons of higher energy are emitted, so the object appears to shift in color from red, to orange, to yellow, and so on. • Of course we have a Law to describe this… Wien’s Law and the Stefan-Boltzmann Law • Wien’s Law: – Hotter bodies emit more strongly at shorter wavelengths • SB Law: – The luminosity of a hot body rises rapidly with temperature Taking the Temperature of Astronomical Objects • Wien’s Law lets us estimate the temperatures of stars easily and fairly accurately • We just need to measure the wavelength (max) at which the star emits the most photons • Then, T= 2.9 ´10 6 K × nm lmax The Stefan-Boltzmann Law • If we know an object’s temperature (T), we can calculate how much energy the object is emitting using the SB law 4 L = sT • is the Stefan-Boltzmann constant, and is equal to 5.6710-8 Watts/m2/K4 • The Sun puts out 64 million watts per square meter – lots of energy! Absorption • If a photon of exactly the right energy (corresponding to the energy difference between orbitals) strikes an electron, that electron will absorb the photon and move into the next higher orbital – The atom is now in an excited state • If the photon is of higher or lower energies, it will not be absorbed – it will pass through as if the atom were not there. • • This process is called absorption If the electron gains enough energy to leave the atom entirely, we say the atom is now ionized, or is an ion. Emission • If an atom drops from one orbital to the next lower one, it must first emit a photon with the same amount of energy as the orbital energy difference. • This is called emission. Frequency • • • • Sometimes it is more convenient to talk about light in terms of frequency, or how fast successive crests pass by a given point You can think of frequency as a measure of how fast you bob up and down as the waves pass. Frequency has units of Hz (Hertz), and is denoted by the symbol Long wavelength light has a low frequency, and short wavelength light has a high frequency • Frequency and wavelength are related by: c ‘c’ is the speed of light. The Electromagnetic Spectrum I • There is more to light than just the visible part of the spectrum – Radio waves are very long wavelength photons (not sound!) with wavelengths longer than a meter or so – Microwaves (yes, the ones we cook with) are at the upper end of the radio part of the spectrum – Infrared wavelengths are just longer in wavelength than the visible spectrum