Radioisotopes
... (Nuclides) of the same chemical element, each having a different atomic mass (mass number) • Isotopes of an element have nuclei with the same number of protons (the same atomic number) but different numbers of neutrons. • Therefore, isotopes have different mass numbers, which give the total number o ...
... (Nuclides) of the same chemical element, each having a different atomic mass (mass number) • Isotopes of an element have nuclei with the same number of protons (the same atomic number) but different numbers of neutrons. • Therefore, isotopes have different mass numbers, which give the total number o ...
CHAPTER 13: Nuclear Interactions and Applications
... A hydrostatic equilibrium exists in the sun between the gravitational attraction tending to contract a star and a gas pressure pushing out due to all the particles. As the lighter nuclides are “burned up” to produce the heavier nuclides, the gravitational attraction succeeds in contracting the star’ ...
... A hydrostatic equilibrium exists in the sun between the gravitational attraction tending to contract a star and a gas pressure pushing out due to all the particles. As the lighter nuclides are “burned up” to produce the heavier nuclides, the gravitational attraction succeeds in contracting the star’ ...
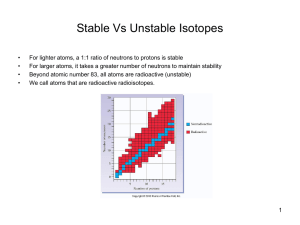
Stable Vs Unstable Isotopes
... Nuclear reactions are accompanied by tremendous energy changes as an unstable isotope spontaneously undergoes changes. ...
... Nuclear reactions are accompanied by tremendous energy changes as an unstable isotope spontaneously undergoes changes. ...
NUCLEAR CHEMISTRY PACKET - Student
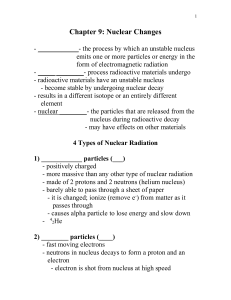
... Nuclear reactions include natural and artificial transmutation, fission, and fusion. (4.4b) There are benefits and risks associated with fission and fusion reactions. (4.4f) Nuclear reactions can be represented by equations that include symbols which represent atomic nuclei (with the mass number and ...
... Nuclear reactions include natural and artificial transmutation, fission, and fusion. (4.4b) There are benefits and risks associated with fission and fusion reactions. (4.4f) Nuclear reactions can be represented by equations that include symbols which represent atomic nuclei (with the mass number and ...
physics - Keith E. Holbert
... For Q > 0, the reaction is exothermic; for Q < 0, reaction is endothermic. From Equation (6), we see that the binding energy (BE) release goes to the kinetic energy of the reaction products. For comparison fission reactions have a Q around 200 MeV, and each fusion reaction releases roughly 10 MeV. E ...
... For Q > 0, the reaction is exothermic; for Q < 0, reaction is endothermic. From Equation (6), we see that the binding energy (BE) release goes to the kinetic energy of the reaction products. For comparison fission reactions have a Q around 200 MeV, and each fusion reaction releases roughly 10 MeV. E ...
2005 Nuclear FRQs - AP Chemistry Olympics
... and write the balanced nuclear reaction for that less. decay process. (c) The neutron/proton ratio in Sr-90 and Cs-137 is (c) Gamma rays are observed during the radioactive too large and they emit beta particles (converting decay of carbon-11. Why is it unnecessary to inneutrons into protons) to low ...
... and write the balanced nuclear reaction for that less. decay process. (c) The neutron/proton ratio in Sr-90 and Cs-137 is (c) Gamma rays are observed during the radioactive too large and they emit beta particles (converting decay of carbon-11. Why is it unnecessary to inneutrons into protons) to low ...