- Physics
... An uncontrolled chain reaction can occur if enough uranium-235 nuclei are in close proximity to each other. If the uranium-235 is in the shape of a sphere about 13 pounds of uranium form a critical mass where a runaway chain reaction (bomb) can occur. I would restate the last paragraph on page 20-4. ...
... An uncontrolled chain reaction can occur if enough uranium-235 nuclei are in close proximity to each other. If the uranium-235 is in the shape of a sphere about 13 pounds of uranium form a critical mass where a runaway chain reaction (bomb) can occur. I would restate the last paragraph on page 20-4. ...
The New Alchemy
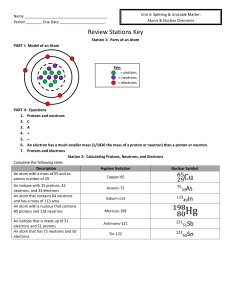
... Protons – one of the parts of an atom. Protons have a (+) charge and are found in the nucleus. Neutrons – one of the parts of an atom. Neutrons have no charge and are found in the nucleus. Nucleus – found in the center of an atom. It contains protons and neutrons. Nuclei is the plural of nucleus. Nu ...
... Protons – one of the parts of an atom. Protons have a (+) charge and are found in the nucleus. Neutrons – one of the parts of an atom. Neutrons have no charge and are found in the nucleus. Nucleus – found in the center of an atom. It contains protons and neutrons. Nuclei is the plural of nucleus. Nu ...
Chapter 3
... As the water boils, heat from the hot stove burner and pan radiates into the surrounding ...
... As the water boils, heat from the hot stove burner and pan radiates into the surrounding ...
Quanta to Quarks part 2 - Connecting-Sharing-and
... collided with heavy atoms such as lead, they would simply bounce off with their original energies. Commonly used moderator materials include ordinary water (in reactors using enriched fuel), heavy water (deuterium oxide D2O), and graphite. Beyond carbon, the atoms are too heavy to do the job efficie ...
... collided with heavy atoms such as lead, they would simply bounce off with their original energies. Commonly used moderator materials include ordinary water (in reactors using enriched fuel), heavy water (deuterium oxide D2O), and graphite. Beyond carbon, the atoms are too heavy to do the job efficie ...
1 AP Chemistry Chapter 21 - The Nucleus: A Chemist`s View 21.1
... a. Inner orbital electron is captured by the nucleus of its own atom b. Electron combines with a proton and a neutron is formed ...
... a. Inner orbital electron is captured by the nucleus of its own atom b. Electron combines with a proton and a neutron is formed ...
AC Geophysical Science Study Guide
... 16. Compare the atomic mass of an atom with the atomic mass of the constituent particles that make up that mass. 17. What is binding energy? How do you determine binding energy? 18. The hydrogen isotope H-3 has a nuclear mass of 3.0155 µ, and the helium isotope He-3 has a nuclear mass of 3.0149 µ. F ...
... 16. Compare the atomic mass of an atom with the atomic mass of the constituent particles that make up that mass. 17. What is binding energy? How do you determine binding energy? 18. The hydrogen isotope H-3 has a nuclear mass of 3.0155 µ, and the helium isotope He-3 has a nuclear mass of 3.0149 µ. F ...