Scientists` Consensus Ideas Atomic Structure and Nuclear Interactions
... a) Electrons are tiny, negatively charged particles that are constantly moving within the empty space that surrounds the nucleus. b) The nucleus contains the positively charged protons and the neutral neutrons. Protons and neutrons are about 2000 times heavier than electrons. 4. According to the qua ...
... a) Electrons are tiny, negatively charged particles that are constantly moving within the empty space that surrounds the nucleus. b) The nucleus contains the positively charged protons and the neutral neutrons. Protons and neutrons are about 2000 times heavier than electrons. 4. According to the qua ...
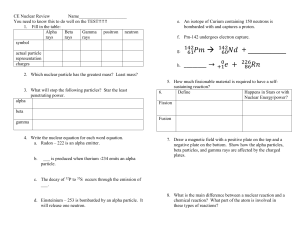
nuclear reactions
... This is not the only possible reaction: a variety of daughter isotopes are produced (As, Br, Sr, Zn, and Zr), some of which are stable, but most of which are radioactive themselves (e.g. as -, + or emitters). These reaction can release 1, 2 or 3 neutrons, and on average 235U fission releases 2 n ...
... This is not the only possible reaction: a variety of daughter isotopes are produced (As, Br, Sr, Zn, and Zr), some of which are stable, but most of which are radioactive themselves (e.g. as -, + or emitters). These reaction can release 1, 2 or 3 neutrons, and on average 235U fission releases 2 n ...
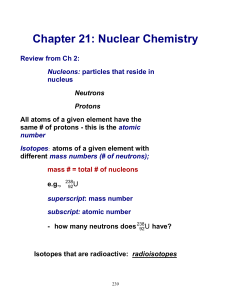
Ch.7 Summary Notes
... Nuclear fusion is a nuclear reaction in which small nuclei combine to produce a larger nucleus. Other subatomic particles as well as energy are released in this process. Fusion occurs at the core of the Sun and other stars where sufficient pressure and high temperatures cause isotopes of hydrogen to ...
... Nuclear fusion is a nuclear reaction in which small nuclei combine to produce a larger nucleus. Other subatomic particles as well as energy are released in this process. Fusion occurs at the core of the Sun and other stars where sufficient pressure and high temperatures cause isotopes of hydrogen to ...
Nuclear Radiation and Decay File
... However, gamma rays cause more damage to biological molecules as they pass through ...
... However, gamma rays cause more damage to biological molecules as they pass through ...