Nuc Chem PP - Liberty Union High School District
... • They will undergo decay • The type of decay depends on the reason for the instability ...
... • They will undergo decay • The type of decay depends on the reason for the instability ...
Name
... types of radioactive decay, such as beta decay 7. Gamma rays are very high energy a. A gamma ray is a high-energy photon emitted by a nucleus during fission and radioactive decay 8. Neutron radioactivity may occur in unstable nucleus a. Neutron emission consists of matter that is emitted from an uns ...
... types of radioactive decay, such as beta decay 7. Gamma rays are very high energy a. A gamma ray is a high-energy photon emitted by a nucleus during fission and radioactive decay 8. Neutron radioactivity may occur in unstable nucleus a. Neutron emission consists of matter that is emitted from an uns ...
Average Atomic Mass
... • The third common type of radiation is gamma radiation or gamma rays. • Gamma rays are high-energy radiation that possess no mass and have no charge. • Gamma rays are denoted by the symbol 00γ. • Gamma rays usually accompany alpha and beta radiation and account for most of the energy lost during th ...
... • The third common type of radiation is gamma radiation or gamma rays. • Gamma rays are high-energy radiation that possess no mass and have no charge. • Gamma rays are denoted by the symbol 00γ. • Gamma rays usually accompany alpha and beta radiation and account for most of the energy lost during th ...
Half Life
... 19. An element that emits rays is said to be contaminated. 20. Unstable isotopes of elements are called radioisotopes. 21. The symbol represents tritium. 22. Gamma rays can be stopped by an aluminum sheet. 23. The change of an atom into a new element is called a chemical change. 24. The first artifi ...
... 19. An element that emits rays is said to be contaminated. 20. Unstable isotopes of elements are called radioisotopes. 21. The symbol represents tritium. 22. Gamma rays can be stopped by an aluminum sheet. 23. The change of an atom into a new element is called a chemical change. 24. The first artifi ...
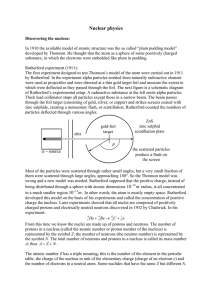
Nuclear physics α −
... them were scattered through large angles, approaching 180°. So the Thomson model was wrong and a new model was needed. Rutherford supposed that the positive charge, instead of being distributed through a sphere with atomic dimensions 10−10 m radius, is all concentrated in a much smaller region 10 −1 ...
... them were scattered through large angles, approaching 180°. So the Thomson model was wrong and a new model was needed. Rutherford supposed that the positive charge, instead of being distributed through a sphere with atomic dimensions 10−10 m radius, is all concentrated in a much smaller region 10 −1 ...
FUSION AND FISSION
... • Large amount of energy generated – 1 million times more than chemical reactions – Nuclear fusion on the sun – Nuclear fission for reactors ...
... • Large amount of energy generated – 1 million times more than chemical reactions – Nuclear fusion on the sun – Nuclear fission for reactors ...