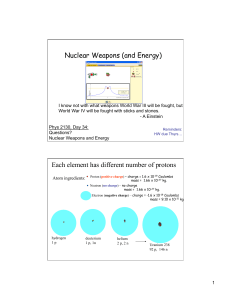
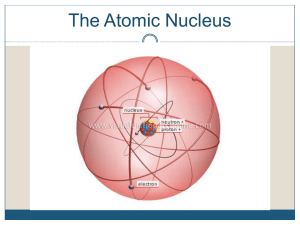
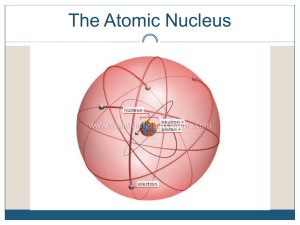
Nuclear Weapons (and Energy) Each element has different number
... This means that after explosion there are a. about 20% fewer atomic nuclei than before with correspondingly fewer total neutrons and protons, b. 20% fewer at. nucl. but about same total neut. and protons. c. about same total neutrons and protons and more atomic nuclei, d. almost no atomic nuclei ...
... This means that after explosion there are a. about 20% fewer atomic nuclei than before with correspondingly fewer total neutrons and protons, b. 20% fewer at. nucl. but about same total neut. and protons. c. about same total neutrons and protons and more atomic nuclei, d. almost no atomic nuclei ...
Radioactivity - Mrs. Sjuts` Science Site
... ! An atom of C-‐14 eventually will decay into N-‐14 with a half-‐life of 5,730 years ! By measuring the amount of C-‐14 in a sample and comparing it to the amount of C-‐12, scientists can ...
... ! An atom of C-‐14 eventually will decay into N-‐14 with a half-‐life of 5,730 years ! By measuring the amount of C-‐14 in a sample and comparing it to the amount of C-‐12, scientists can ...
Chapter 1 Learning Objective Summary
... Chemical reactions involve the gain, loss, or sharing of the outer electrons, whereas nuclear reactions involve changes to the composition of the nucleus. This means that alchemy is possible (though not economical!), because transmutation of one element into another can be accomplished via radioacti ...
... Chemical reactions involve the gain, loss, or sharing of the outer electrons, whereas nuclear reactions involve changes to the composition of the nucleus. This means that alchemy is possible (though not economical!), because transmutation of one element into another can be accomplished via radioacti ...
Nuclear Reactions Review
... nuclear energy as a power source? a.Nuclear energy produces less energy than the burning of coal. b.Nuclear energy produces air pollution. c.Nuclear waste must be safely stored. d.The fuel source is very limited. ...
... nuclear energy as a power source? a.Nuclear energy produces less energy than the burning of coal. b.Nuclear energy produces air pollution. c.Nuclear waste must be safely stored. d.The fuel source is very limited. ...