21J 2011 The Polywell Nuclear Reactor Website July 4, 2011
... The mass numbers given in the table are the known stable isotopes, which do not break down spontaneously. Unstable isotopes are not shown because there are too many of them. When unstable isotopes break down into new isotopes, they usually emit alpha, beta, or gamma radiation. It says “none” above U ...
... The mass numbers given in the table are the known stable isotopes, which do not break down spontaneously. Unstable isotopes are not shown because there are too many of them. When unstable isotopes break down into new isotopes, they usually emit alpha, beta, or gamma radiation. It says “none” above U ...
Unit 2: The Atom
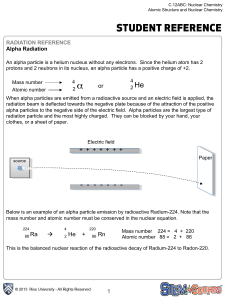
... •Alpha decay is how elements greater than Bismuth try to become stable. •They will emit an alpha particle (2 neutrons and 2 protons) to try to become stable. •Alpha reactions will always have He on the right side! •To balance: write the upper and lower equations! ...
... •Alpha decay is how elements greater than Bismuth try to become stable. •They will emit an alpha particle (2 neutrons and 2 protons) to try to become stable. •Alpha reactions will always have He on the right side! •To balance: write the upper and lower equations! ...
Chapter 9 Nuclear Radiation
... speed of light, 3.0 108 m/s. Using this equation, a small amount of mass is multiplied by the speed of light squared, resulting in a large amount of energy. Fission of 1 g U-235 produces same energy as 3 tons of coal. ...
... speed of light, 3.0 108 m/s. Using this equation, a small amount of mass is multiplied by the speed of light squared, resulting in a large amount of energy. Fission of 1 g U-235 produces same energy as 3 tons of coal. ...
CH_8_nucleus_new
... Rutherford found that the only way to explain the deflections was to picture an atom with a tiny nucleus in which positive charge existed and nearly all the mass existed; And the electrons were some distance away from the nucleus. In other words, AN ATOM IS MOSTLY EMPTY SPACE. ...
... Rutherford found that the only way to explain the deflections was to picture an atom with a tiny nucleus in which positive charge existed and nearly all the mass existed; And the electrons were some distance away from the nucleus. In other words, AN ATOM IS MOSTLY EMPTY SPACE. ...
chap7_nucleus
... Rutherford found that the only way to explain the deflections was to picture an atom with a tiny nucleus in which positive charge existed and nearly all the mass existed; And the electrons were some distance away from the nucleus. In other words, AN ATOM IS MOSTLY EMPTY SPACE. ...
... Rutherford found that the only way to explain the deflections was to picture an atom with a tiny nucleus in which positive charge existed and nearly all the mass existed; And the electrons were some distance away from the nucleus. In other words, AN ATOM IS MOSTLY EMPTY SPACE. ...
6.2 Atomic Nucleus Stability and Isotopes
... Neutrons and Protons: Made up of Quarks Neutrons and protons are particles called baryons. A baryon is made up of 3 particles called quarks. There are six kinds of quarks, each having a fractional charge in relation to the charge of a proton. The most common baryons are neutrons and protons. Proton ...
... Neutrons and Protons: Made up of Quarks Neutrons and protons are particles called baryons. A baryon is made up of 3 particles called quarks. There are six kinds of quarks, each having a fractional charge in relation to the charge of a proton. The most common baryons are neutrons and protons. Proton ...
1412-PracticeExam4
... Which of these species are structural isomers of C6H14? A. I and II B. I and III C. II and III D. II and IV E. III and IV Which of these species is an aromatic compound? A. C2H2 B. C6H12 C. C6H4Br2 D. C5H10 E. C2H4Br2 The name for the compound with the formula CH3CH2CH2CH2OH is A. ...
... Which of these species are structural isomers of C6H14? A. I and II B. I and III C. II and III D. II and IV E. III and IV Which of these species is an aromatic compound? A. C2H2 B. C6H12 C. C6H4Br2 D. C5H10 E. C2H4Br2 The name for the compound with the formula CH3CH2CH2CH2OH is A. ...
notes ch 39 1st half Atomic Nucleus and Radioactivity
... • The strong force is only strong enough to hold things together at very small distances. • When the number of protons increases in a nucleus, then the number of neutrons needed to hold the nucleus together must get larger too. • At small atomic numbers, the number of neutrons may be equal or slight ...
... • The strong force is only strong enough to hold things together at very small distances. • When the number of protons increases in a nucleus, then the number of neutrons needed to hold the nucleus together must get larger too. • At small atomic numbers, the number of neutrons may be equal or slight ...
FUSION-FISSION HYBRID REACTOR STUDIES FOR THE STRAIGHT FIELD LINE MIRROR
... In Monte Carlo simulations for the neutrons13, the geometry and materials in the fission mantle is designed to have an initial neutron multiplicity of keff = 0.97. This number is selected with the expectation that the reactor would remain in a subcritical state even in “worst case scenarios” 6,12. T ...
... In Monte Carlo simulations for the neutrons13, the geometry and materials in the fission mantle is designed to have an initial neutron multiplicity of keff = 0.97. This number is selected with the expectation that the reactor would remain in a subcritical state even in “worst case scenarios” 6,12. T ...
Unit 3 - Princeton High School
... More than 2000 years ago, a Greek philosopher named _____________ proposed the existence of very small, indivisible particles, each of which was called a(n) _____________. The theory that such particles existed was supported much later, by _____________ who proposed, in his law of _______________ __ ...
... More than 2000 years ago, a Greek philosopher named _____________ proposed the existence of very small, indivisible particles, each of which was called a(n) _____________. The theory that such particles existed was supported much later, by _____________ who proposed, in his law of _______________ __ ...