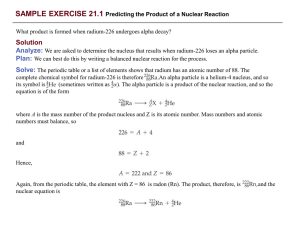
Nuclear and Thermal Physics
... characteristic of nuclei having a large proportion of neutrons (neutron changes to proton). + emission is characteristic of nuclei having a large proportion of protons. The ejected electrons come from the nucleus rather than from the electron cloud, and yet do not exist as electrons while in the n ...
... characteristic of nuclei having a large proportion of neutrons (neutron changes to proton). + emission is characteristic of nuclei having a large proportion of protons. The ejected electrons come from the nucleus rather than from the electron cloud, and yet do not exist as electrons while in the n ...
Atomic Origins: Chapter Problems Big Bang Class Work 1. How old
... This element has a radioactive isotope (X6) with a half-life of 80.4 milliseconds. How long will it take for 60 g of the substance to decay to 3.75 g? e. This element was generated through a fusion reaction. Where in the universe did this fusion reaction occur? 3. Hydrogen has two stable isotopes, h ...
... This element has a radioactive isotope (X6) with a half-life of 80.4 milliseconds. How long will it take for 60 g of the substance to decay to 3.75 g? e. This element was generated through a fusion reaction. Where in the universe did this fusion reaction occur? 3. Hydrogen has two stable isotopes, h ...
Module P9.3 Nuclear fission and fusion and radiation hazards
... successful, will produce nuclear power with much less radioactive hazard than existing nuclear fission reactors. In Section 3 we describe the underlying physical principles of power generation by fusion, including the use of deuterium and tritium as fuels, and the need for a very high temperature pl ...
... successful, will produce nuclear power with much less radioactive hazard than existing nuclear fission reactors. In Section 3 we describe the underlying physical principles of power generation by fusion, including the use of deuterium and tritium as fuels, and the need for a very high temperature pl ...
nuclear physics - Sakshi Education
... B: A good moderator must be light (low atomic weight) must be capable of scattering neutrons with a high probability, but should not absorb neutrons. Therefore Beryllium is not suitable for moderator ...
... B: A good moderator must be light (low atomic weight) must be capable of scattering neutrons with a high probability, but should not absorb neutrons. Therefore Beryllium is not suitable for moderator ...
do physics online from quanta to quarks radioactivity
... beta or gamma radioactive emissions hit living cells they cause ionize atoms. They can kill cells directly or cause genetic damage to the DNA molecules. High radiation doses will cause burn effects as well as kill the damaged cells. However, low doses don't kill the cells, but if the cells are genet ...
... beta or gamma radioactive emissions hit living cells they cause ionize atoms. They can kill cells directly or cause genetic damage to the DNA molecules. High radiation doses will cause burn effects as well as kill the damaged cells. However, low doses don't kill the cells, but if the cells are genet ...
Radioactive Decay
... _______________ (α): two protons and two neutrons bound together, emitted during some types of radioactive ...
... _______________ (α): two protons and two neutrons bound together, emitted during some types of radioactive ...
Atomic Energy for Military Purposes
... observed in ordinary chemical combustion. We now believe that the heat given off in such a combustion has mass associated with it, but this mass is so small that it cannot be detected by the most sensitive balances available. (It is of the order of a few billionths of a gram per mole.) In the second ...
... observed in ordinary chemical combustion. We now believe that the heat given off in such a combustion has mass associated with it, but this mass is so small that it cannot be detected by the most sensitive balances available. (It is of the order of a few billionths of a gram per mole.) In the second ...
31.1 Nuclear Structure
... It is well known that lead and oxygen contain different atoms and that the density of solid lead is much greater than gaseous oxygen. Using the equation, decide whether the density of the nucleus in a lead atom is greater than, approximately equal to, or less than that in an oxygen atom. ...
... It is well known that lead and oxygen contain different atoms and that the density of solid lead is much greater than gaseous oxygen. Using the equation, decide whether the density of the nucleus in a lead atom is greater than, approximately equal to, or less than that in an oxygen atom. ...