* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download 06_Medical equipment based on ionizing radiation principle
Survey
Document related concepts
Isotopic labeling wikipedia , lookup
Nuclear fission product wikipedia , lookup
Radiation therapy wikipedia , lookup
Nuclear binding energy wikipedia , lookup
Fallout shelter wikipedia , lookup
Gamma spectroscopy wikipedia , lookup
Valley of stability wikipedia , lookup
Nuclear transmutation wikipedia , lookup
Radioactive decay wikipedia , lookup
Ionizing radiation wikipedia , lookup
Technetium-99m wikipedia , lookup
Transcript
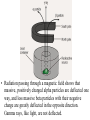
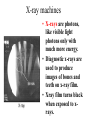
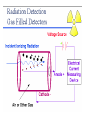
Interaction ionizing radiation with biological tissue. Basic dosimetry. Radioactivity • The word radioactivity was first used by Marie Curie in 1898. • She used the word radioactivity to describe the property of certain substances to give off invisible “radiations” that could be detected by films. Radioactivity • Scientists quickly learned that there were three different kinds of radiation given off by radioactive materials. – Alpha rays – Beta rays – Gamma rays • The scientists called them “rays” because the radiation carried energy and moved in straight lines, like light rays. Radioactivity • We now know that radioactivity comes from the nucleus of the atom. • If the nucleus has too many neutrons, or is unstable for any other reason, the atom undergoes radioactive decay. • The word decay means to "break down." Radioactivity • In alpha decay, the nucleus ejects two protons and two neutrons. • Beta decay occurs when a neutron in the nucleus splits into a proton and an electron. • Gamma decay is not truly a decay reaction in the sense that the nucleus becomes something different. Radiation Radiation: The process of emitting energy in the form of waves or particles. Where does radiation come from? Radiation is generally produced when particles interact or decay. A large contribution of the radiation on earth is from the sun (solar) or from radioactive isotopes of the elements (terrestrial). Radiation is going through you at this very moment! http://www.atral.com/U238.html Isotopes What’s an isotope? Two or more varieties of an element having the same number of protons but different number of neutrons. Certain isotopes are “unstable” and decay to lighter isotopes or elements. Deuterium and tritium are isotopes of hydrogen. In addition to the 1 proton, they have 1 and 2 additional neutrons in the nucleus respectively*. Another prime example is Uranium 238, or just 238U. • Introduction – Henri Becquerel discovered radioactivity in 1896 • Becquerel named the emission of invisible radiation from uranium ore radioactivity. • Radioactive materials was the name given to materials that gave off this invisible radiation. • Radioactivity was discovered by Henri Becquerel when he exposed a light-tight photographic plate to a radioactive mineral, then developed the plate. (A) A photographic film is exposed to an uranite ore sample. (B) The film, developed normally after a four-day exposure to uranite. Becquerel found an image like this one and deduced that the mineral gave off invisible radiation that he called radioactivity. – Ernest Rutherford later discovered that there were three kinds of radioactivity. • Alpha particles () is a helium nucleus (2 protons and 2 neutrons) • A beta particle () is a high energy electron • A gamma ray () is electromagnetic radiation with a very short wavelength. • Radiation passing through a magnetic field shows that massive, positively charged alpha particles are deflected one way, and less massive beta particles with their negative charge are greatly deflected in the opposite direction. Gamma rays, like light, are not deflected. – Radioactivity is the spontaneous emission of particles or energy from an atomic nucleus as it disintegrates. – Radioactive decay is the spontaneous disintegration of decomposition of a nucleus. • Nuclear Equations – The two subatomic particles that occur in the nucleus, the proton and the neutron, are called nucleons. • The number of protons is the atomic number which determines the identity of the element. • The number of protons and neutrons determines the atomic mass of the element. • Different isotopes of an element have the same atomic number (same number of protons) but different atomic masses (different number of neutrons) Radioactivity By the end of the 1800s, it was known that certain isotopes emit penetrating rays. Three types of radiation were known: 1) Alpha particles () 2) Beta particles () 3) Gamma-rays () Where do these particles come from ? These particles generally come from the nuclei of atomic isotopes which are not stable. The decay chain of Uranium produces all three of these forms of radiation. Let’s look at them in more detail… Discovery of Radioactivity • Antoine Henri Becquerel (1852-1908) – Noticed the fogging of photographic plate by uranium crystals • Pierre Curie (1859-1906), Marie Curie (18671934) – Further studies of uranium and discovery of polonium and radium. Marie received two Nobel prizes. She died from the effects of radiation doses received during her experiments • Ernest Rutherford (1871-1937) – His understanding of atomic structure helped us understand the role of the nucleus. He defined many of the terms used to discuss radioactivity today Note: This is the atomic weight, which is the number of protons plus neutrons Alpha Particles () Radium Radon R226 Rn222 88 protons 138 neutrons n p p n + 86 protons 136 neutrons (4He) 2 protons 2 neutrons The alpha-particle () is a Helium nucleus. It’s the same as the element Helium, with the electrons stripped off ! Net effect is loss of 4 in mass number and loss of 2 in atomic number. Beta Particles () Carbon C14 Nitrogen N14 6 protons 8 neutrons 7 protons 7 neutrons + eelectron (beta-particle) We see that one of the neutrons from the C14 nucleus “converted” into a proton, and an electron was ejected. The remaining nucleus contains 7p and 7n, which is a nitrogen nucleus. In symbolic notation, the following process occurred: np+e (+n) Yes, the same neutrino we saw previously Gamma particles () In much the same way that electrons in atoms can be in an excited state, so can a nucleus. Neon Ne20 10 protons 10 neutrons (in excited state) Neon Ne20 + 10 protons 10 neutrons (lowest energy state) gamma A gamma is a high energy light particle. It is NOT visible by your naked eye because it is not in the visible part of the EM spectrum. Gamma Rays Neon Ne20 Neon Ne20 + The gamma from nuclear decay is in the X-ray/ Gamma ray part of the EM spectrum (very energetic!) A. Radioactive Decay Types of Radioactive Decay • Positron production • Positron – particle with same mass as an electron but with a positive charge (antimatter version of an electron) – Examples • Net effect is to change a proton to a neutron. A. Radioactive Decay Types of Radioactive Decay • Electron capture • Inner orbital electron is captured. New nucleus formed. Neutrino and gamma ray produced 201 Hg 80 + 0-1e → 20179Au + ν + 00γ • Net effect is to change a proton to a neutron How do these particles differ ? Particle Mass* (MeV/c2) Charge Gamma () 0 0 Beta () ~0.5 -1 Alpha () ~3752 +2 * m = E / c2 Rate of Decay Beyond knowing the types of particles which are emitted when an isotope decays, we also are interested in how frequently one of the atoms emits this radiation. A very important point here is that we cannot predict when a particular entity will decay. We do know though, that if we had a large sample of a radioactive substance, some number will decay after a given amount of time. Some radioactive substances have a very high “rate of decay”, while others have a very low decay rate. To differentiate different radioactive substances, we look to quantify this idea of “decay rate” Radioactive Decay Conservation of Mass Number and Charge Number − both are retained in a nuclear reaction − sum of both from the “reactants and products” are constant Band of Stability Black squares indicate stable nuclei. Decay occurs to move isotopes towards the black line Nuclear Transformations • Nuclear Transformation – forced change of one element to another • Bombard elements with particles • Transuranium elements – elements with atomic numbers greater than 92 which have been synthesized UUO Detection of Radioactivity and the Concept of Half-life • Geiger-Muller counter – instrument which measures radioactive decay by registering the ions and electrons produced as a radioactive particle passes through a gasfilled chamber Detection of Radioactivity and the Concept of Half-life • Scintillation counter instrument which measures the rate of radioactive decay by sensing flashes of light that the radiation produces in the detector X-ray machines • X-rays are photons, like visible light photons only with much more energy. • Diagnostic x-rays are used to produce images of bones and teeth on x-ray film. • Xray film turns black when exposed to xrays. X-ray machines • Therapeutic x-rays are used to destroy diseased tissue, such as cancer cells. • Low levels of x-rays do not destroy cells, but high levels do. • The beams are made to overlap at the place where the doctor wants to destroy diseased cells. CAT scan • The advent of powerful computers has made it possible to produce threedimensional images of bones and other structures within the body. • To produce a CAT scan, computerized axial tomography, a computer controls an x-ray machine as it takes pictures of the body from different angles. CAT scan • People who work with radiation use radiation detectors to tell when radiation is present and to measure its intensity. • The Geiger counter is a type of radiation detector invented to measure x-rays and other ionizing radiation, since they are invisible to the naked eye. Detection of Radioactivity and the Concept of Half-life • Half-life – time required for half of the original sample of radioactive nuclides to decay Decay of a Radioactive Element Half of the radioactive parent atoms decay after one half-life. Half of the remainder decay after another half-life and so on…….. Half-life activity Fundamental law of radioactive decay • Each nucleus has a fixed probability of decaying per unit time. Nothing affects this probability (e.g., temperature, pressure, bonding environment, etc.) [exception: very high pressure promotes electron capture slightly] • This is equivalent to saying that averaged over a large enough number of atoms the number of decays per unit time is proportional to the number of atoms present. • Therefore in a closed system: dN N dt (Equation 3.1) – N = number of parent nuclei at time t – = decay constant = probability of decay per unit time (units: s–1) • To get time history of number of parent nuclei, integrate 3.1: N (t ) Noe t – No = initial number of parent nuclei at time t = 0. (3.2) 37 Definitions • The mean life t of a parent nuclide is given by the number present divided by the removal rate (recall this later when we talk about residence time): N 1 t N – This is also the “e-folding” time of the decay: N(t ) No e t Noe 1 No e • The half life t1/2 of a nucleus is the time after which half the parent remains: No ln 2 .693 t1/2 t ln2 (3.3) N(t1/ 2 ) Noe t1/2 1/ 2 2 • The activity is decays per unit time, denoted by parentheses: ( N ) N (3.4) 38 Decay of parent 0 ln(N)–ln(No) Activity No No 2 No e 0 t 1/2 t 3t 2t 4t -1 -2 slope = -1 -3 -4 -5 5t 0 time t 1/2 t 2t 3t 4t 5t time Some dating schemes only consider measurement of parent nuclei because initial abundance is somehow known. • 14C-14N: cosmic rays create a roughly constant atmospheric 14C inventory, so that living matter has a roughly constant 14C/C ratio while it exchanges CO2 with the environment through photosynthesis or diet. After death this 14C decays with half life 5730 years. Hence even through the daughter 14N is not retained or measured, age is calculated using: 14 t 1 ( C) / C ln 14 14 ( C) / C o 39 Half-life is the time required for the quantity of a radioactive material to be reduced to one-half its original value. All radionuclides have a particular half-life, some of which a very long, while other are extremely short. For example, uranium-238 has such a long half life, 4.5x109 years, that only a small fraction has decayed since the earth was formed. In contrast, carbon-11 has a half-life of only 20 minutes. Since this nuclide has medical applications, it has to be created where it is being used so that enough will be present to conduct medical studies. When given a certain amount of radioactive material, it is customary to refer to the quantity based on its activity rather than its mass. The activity is simply the number of disintegrations or transformations the quantity of material undergoes in a given period of time. The two most common units of activity are the Curie and the Becquerel. The Curie is named after Pierre Curie for his and his wife Marie's discovery of radium. One Curie is equal to 3.7x1010 disintegrations per second. A newer unit of activity if the Becquerel named for Henry Becquerel who is credited with the discovery of radioactivity. One Becquerel is equal to one disintegration per second. It is obvious that the Curie is a very large amount of activity and the Becquerel is a very small amount. To make discussion of common amounts of radioactivity more convenient, we often talk in terms of milli and microCuries or kilo and MegaBecquerels. Common Radiation Units – SI Gray (Gy) - to measure absorbed dose ... the amount of energy actually absorbed in some material, and is used for any type of radiation and any material (does not't describe the biological effects of the different radiations) Gy = J / kg (one joule of energy deposited in one kg of a material) Sievert (Sv) - to derive equivalent dose ... the absorbed dose in human tissue to the effective biological damage of the radiation Sv = Gy x Q (Q = quality factor unique to the type of incident radiation) Becquerel (Bq) - to measure a radioactivity … the quantity of a radioactive material that have 1 transformations /1s Bq = one transformation per second, there are 3.7 x 1010 Bq in one curie. __________________________________________________________________________________ Roentgen (R) - to measure exposure but only to describe for gamma and X-rays, and only in air. R = depositing in dry air enough energy to cause 2.58E-4 coulombs per kg Rad (radiation absorbed dose) - to measure absorbed dose Rem (roentgen equivalent man) - to derive equivalent dose related the absorbed dose in human tissue to the effective biological damage of the radiation. Curie (Ci) - to measure radioactivity. One curie is that quantity of a radioactive material that will have 37,000,000,000 transformations in one second. 3.7 x 1010 Bq • • • • • • Since we cannot see, smell or taste radiation, we are dependent on instruments to indicate the presence of ionizing radiation. The most common type of instrument is a gas filled radiation detector. This instrument works on the principle that as radiation passes through air or a specific gas, ionization of the molecules in the air occur. When a high voltage is placed between two areas of the gas filled space, the positive ions will be attracted to the negative side of the detector (the cathode) and the free electrons will travel to the positive side (the anode). These charges are collected by the anode and cathode which then form a very small current in the wires going to the detector. By placing a very sensitive current measuring device between the wires from the cathode and anode, the small current measured and displayed as a signal. The more radiation which enters the chamber, the more current displayed by the instrument. Many types of gas-filled detectors exist, but the two most common are the ion chamber used for measuring large amounts of radiation and the Geiger-Muller or GM detector used to measure very small amounts of radiation. The second most common type of radiation detecting instrument is the scintillation detector. The basic principle behind this instrument is the use of a special material which glows or “scintillates” when radiation interacts with it. The most common type of material is a type of salt called sodium-iodide. The light produced from the scintillation process is reflected through a clear window where it interacts with device called a photomultiplier tube. The first part of the photomultiplier tube is made of another special material called a photocathode. The photocathode has the unique characteristic of producing electrons when light strikes its surface. These electrons are then pulled towards a series of plates called dynodes through the application of a positive high voltage. When electrons from the photocathode hit the first dynode, several electrons are produced for each initial electron hitting its surface. This “bunch” of electrons is then pulled towards the next dynode, where more electron “multiplication” occurs. The sequence continues until the last dynode is reached, where the electron pulse is now millions of times larger then it was at the beginning of the tube. At this point the electrons are collected by an anode at the end of the tube forming an electronic pulse. The pulse is then detected and displayed by a special instrument. Scintillation detectors are very sensitive radiation instruments and are used for special environmental surveys and as laboratory instruments. Terms Related to Radiation Dose Chronic dose … means a person received a radiation dose over a long period of time. Acute dose … means a person received a radiation dose over a short period of time. Somatic effects … are effects from some agent, like radiation that are seen in the individual who receives the agent. Genetic effects … are effects from some agent, that are seen in the offspring of the individual who received the agent. The agent must be encountered pre-conception. Teratogenic effects … are effects from some agent, that are seen in the offspring of the individual who received the agent. The agent must be encountered during the gestation period. Stochastic effects … are effects that occur on a random basis with its effect being independent of the size of dose. The effect typically has no threshold and is based on probabilities, with the chances of seeing the effect increasing with dose. Cancer is a stochastic effect. Non-stochastic effect … are effects that can be related directly to the dose received. The effect is more severe with a higher dose, i.e., the burn gets worse as dose increases. It typically has a threshold, below which the effect will not occur. A skin burn from radiation is a non-stochastic effect. PET In clinical applications, a very small amount of labelled compound (called radiopharmaceutical or radiotracer) is introduced into the patient usually by intravenous injection and after an appropriate uptake period, the concentration of tracer in tissue is measured by the scanner. During its decay process, the radionuclide emits a positron which, after travelling a short distance (3-5 mm), encounters an electron from the surrounding environment. The two particles combine and "annihilate" each other resulting in the emission in opposite directions of two gamma rays of 511 keV each. The image acquisition is based on the external detection in coincidence of the emitted gamma-rays, and a valid annihilation event requires a coincidence within 12 nanoseconds between two detectors on opposite sides of the scanner. For accepted coincidences, lines of response connecting the coincidence detectors are drawn through the object and used in the image reconstruction. Any scanner requires that the radioisotope, in the field of view, does not redistribute during the scan. A tissue attenuation correction is performed by recording a short transmission scan using gammarays from three radioactive (Germanium-68/Gallium-68) rotating rod sources. NMR (MRI) Nuclear Magnetic Resonance (NMR) Spectroscopy In NMR, EM radiation is used to "flip" the alignment of nuclear spins from the low energy spin aligned state to the higher energy spin opposed state. The energy required for this transition depends on the strength of the applied magnetic field (see below) but in is small and corresponds to the radio frequency range of the EM spectrum. Nuclei with an odd mass or odd atomic number have "nuclear spin" (in a similar fashion to the spin of electrons). This includes 1H and 13C (but not 12C). The spins of nuclei are sufficiently different that NMR experiments can be sensitive for only one particular isotope of one particular element. The NMR behaviour of 1H and 13C nuclei has been exploited by organic chemist since they provide valuable information that can be used to deduce the structure of organic compounds. These will be the focus of our attention. Since a nucleus is a charged particle in motion, it will develop a magnetic field. 1H and 13C have nuclear spins of 1/2 and so they behave in a similar fashion to a simple, tiny bar magnet. In the absence of a magnetic field, these are randomly oriented but when a field is applied they line up parallel to the applied field, either spin aligned or spin opposed. The more highly populated state is the lower energy spin state spin aligned situation. Two schematic representations of these arrangements are shown below: Computed Tomography Imaging (CT Scan, CAT Scan) Computed Tomography is based on the x-ray principal: as x-rays pass through the body they are absorbed or attenuated (weakened) at differing levels creating a matrix or profile of x-ray beams of different strength. This x-ray profile is registered on film, thus creating an image. In the case of CT, the film is replaced by a banana shaped detector which measures the x-ray profile. Computed Tomography (CT) imaging, also known as "CAT scanning" (Computed Axial Tomography), combines the use of a digital computer together with a rotating x-ray device to create detailed cross sectional images or "slices" of the different organs and body parts such as the lungs, liver, kidneys, pancreas, pelvis, extremities, brain, spine, and blood vessels. For many patients, CT can be performed on an outpatient basis High resolution axial CT image of the inner ears and sinuses. A large polyp in the right sinus (arrow) can be seen Among the various imaging techniques such as MR and x-ray, CT has the unique ability to image a combination of soft tissue, bone, and blood vessels. For example, conventional x-ray imaging of the head can only show the dense bone structures of the skull. X-ray angiography of the head only depicts the blood vessels of the head and neck and not the soft brain-tissue. Magnetic resonance (MR) imaging does an excellent job of showing soft tissue and blood vessels, but MR does not give as much detail of bony structures such as the skull. CT images of the head allow physicians to see soft-tissue anatomic structures like the brain's ventricles or gray and white matter. Physician then can selectively "window" the digital CT images on the computer monitor to look at the soft tissue, then the bone and then the blood vessels, as needed. NUCLEAR MEDICINE - highly specialized on detection and diagnosis of functional disturbances, the morphology is mostly secondary Main advantages ot this method are : Radioactive isotopes introduced into an organism are distinguishable by their radiation from the atoms already present. This permits the relatively simple acquisition of information about the dynamics of processes of uptake, incorporation, exchange, secretion, etc. The tracer method is extremely sensitive. In principle even the presence of only one atom can be detected. The high sensitivity allows the study of various processes with amounts of substances so small that they have no influence on the life processes. “In vivo methods” 1) labeled molecules and compounds, which behave virtually identically to the unlabelled ones in the various chemical, biochemical and biological processes 2) radioactive isotopes form compounds in the same way like as the stable isotopes 3) isotopes disclose their presence by their radiation, and thus their movement and fate can be traced For these purposes are used radionuclides that emit electromagnetic waves ( rays) but don’t emit any particle (, or neutron). 4) Radiopharmaceuticals the most widely used radioisotope is Tc, with a half-life of six hours. activity in the organ can then be studied either as a two dimensional picture or, with a special technique called tomography, as a three dimensional picture (SPECT, PET) Thank you for your attention