Name - TeacherWeb
... a. Nonmetals are poor conductors of heat and electric current. b. Many nonmetals are gases at room temperature. c. Some nonmetals are extremely reactive and others hardly react at all. d. Nonmetals that are solids tend to be malleable. 17. Across a period from left to right, the elements become less ...
... a. Nonmetals are poor conductors of heat and electric current. b. Many nonmetals are gases at room temperature. c. Some nonmetals are extremely reactive and others hardly react at all. d. Nonmetals that are solids tend to be malleable. 17. Across a period from left to right, the elements become less ...
Chem 1411 Chapt2
... Molecular (covalent)- Consists of non-metals only. HCl, N2O4, C3H6O, C6H12O6 Note- All compounds can be molecules; not all molecules can be compounds. Ions- Are chemical species that have a net charge. Monatomic- cations: K+, Na+, Mg+2, Al+3 Anions: Cl-, O2-, BrThe monatomic ions like to take charge ...
... Molecular (covalent)- Consists of non-metals only. HCl, N2O4, C3H6O, C6H12O6 Note- All compounds can be molecules; not all molecules can be compounds. Ions- Are chemical species that have a net charge. Monatomic- cations: K+, Na+, Mg+2, Al+3 Anions: Cl-, O2-, BrThe monatomic ions like to take charge ...
Glossary of Terms Used in Articles about the Hydrogen Economy at
... A fundamental particle of matter, an electron can exists as a component of an atom or in the free state. Each electron carries one unit of negative charge and has a very small mass as compared with that of a neutron or proton. Fuel reforming Reforming is decomposition (cracking) of hydrocarbon gases ...
... A fundamental particle of matter, an electron can exists as a component of an atom or in the free state. Each electron carries one unit of negative charge and has a very small mass as compared with that of a neutron or proton. Fuel reforming Reforming is decomposition (cracking) of hydrocarbon gases ...
AP Chemistry Summer Assignment
... a. A compound wit the molecular formula C6H6 has the same simplest formula. b. The mass percent of copper in CuO is less than in Cu2O. c. The limiting reactant is the one present in the smallest number of grams. d. Since C3H6O3 and C6H12O6 reduce to the same formula, they represent the ...
... a. A compound wit the molecular formula C6H6 has the same simplest formula. b. The mass percent of copper in CuO is less than in Cu2O. c. The limiting reactant is the one present in the smallest number of grams. d. Since C3H6O3 and C6H12O6 reduce to the same formula, they represent the ...
AP Chemistry Summer Assignment
... a. A compound wit the molecular formula C6H6 has the same simplest formula. b. The mass percent of copper in CuO is less than in Cu2O. c. The limiting reactant is the one present in the smallest number of grams. d. Since C3H6O3 and C6H12O6 reduce to the same formula, they represent the ...
... a. A compound wit the molecular formula C6H6 has the same simplest formula. b. The mass percent of copper in CuO is less than in Cu2O. c. The limiting reactant is the one present in the smallest number of grams. d. Since C3H6O3 and C6H12O6 reduce to the same formula, they represent the ...
AP Chemistry Summer Assignment
... a. A compound wit the molecular formula C6H6 has the same simplest formula. b. The mass percent of copper in CuO is less than in Cu2O. c. The limiting reactant is the one present in the smallest number of grams. d. Since C3H6O3 and C6H12O6 reduce to the same formula, they represent the ...
... a. A compound wit the molecular formula C6H6 has the same simplest formula. b. The mass percent of copper in CuO is less than in Cu2O. c. The limiting reactant is the one present in the smallest number of grams. d. Since C3H6O3 and C6H12O6 reduce to the same formula, they represent the ...
standard sample test
... If there is a limiting reagent, there is also a substance in excess. The limiting reagent controls the reaction. If the limiting reagent is a gas, then it does not control the reaction. The mass of all the products depend on the mass of the limiting reagent. ...
... If there is a limiting reagent, there is also a substance in excess. The limiting reagent controls the reaction. If the limiting reagent is a gas, then it does not control the reaction. The mass of all the products depend on the mass of the limiting reagent. ...
AP Chemistry Summer Assignment
... a. A compound wit the molecular formula C6H6 has the same simplest formula. b. The mass percent of copper in CuO is less than in Cu2O. c. The limiting reactant is the one present in the smallest number of grams. d. Since C3H6O3 and C6H12O6 reduce to the same formula, they represent the ...
... a. A compound wit the molecular formula C6H6 has the same simplest formula. b. The mass percent of copper in CuO is less than in Cu2O. c. The limiting reactant is the one present in the smallest number of grams. d. Since C3H6O3 and C6H12O6 reduce to the same formula, they represent the ...
ionic and covalent bonding - Atomic Theory and Periodic Table
... The formula of an ionic compound is actually the _______________ in which the ions are present. This can be easily determined because the overall charge on an ionic compound must neutral be _______________. As this formula gives a ratio rather than actual numbers of ions, it is empirical called an _ ...
... The formula of an ionic compound is actually the _______________ in which the ions are present. This can be easily determined because the overall charge on an ionic compound must neutral be _______________. As this formula gives a ratio rather than actual numbers of ions, it is empirical called an _ ...
Ex. 06 Answer
... would probably have a relative atomic mass between 16 and 20. The second missing element # (after R and D) would be a colourless gas, with nil electrical conductivity and high reactivity. It would probably have a relative atomic mass between 24 and ...
... would probably have a relative atomic mass between 16 and 20. The second missing element # (after R and D) would be a colourless gas, with nil electrical conductivity and high reactivity. It would probably have a relative atomic mass between 24 and ...
Ionic Bonding
... 1. Describe how we can use electronegativity values to predict the types of bonds that will form within a compound. 2. Which type of bond—ionic or covalent—will form between each of the following pairs of atoms? Of the covalent bonds, indicate which would be the most polar. (a) H and Cl (b) Si and O ...
... 1. Describe how we can use electronegativity values to predict the types of bonds that will form within a compound. 2. Which type of bond—ionic or covalent—will form between each of the following pairs of atoms? Of the covalent bonds, indicate which would be the most polar. (a) H and Cl (b) Si and O ...
AP Chemistry Summer Assignment
... g. Potassium Chloride. 42. The hormone, thyroxine is secreted by the thyroid gland, and has the formula: C15H17NO4I4. How many milligrams of Iodine can be extracted from 15.0 Grams of thyroxine? 43. Determine the formula weight for the following: a. N2O5 b. CuSO4 C. Ca(HCO3)2 d. CaSO4 . 2 H2O 44. Wr ...
... g. Potassium Chloride. 42. The hormone, thyroxine is secreted by the thyroid gland, and has the formula: C15H17NO4I4. How many milligrams of Iodine can be extracted from 15.0 Grams of thyroxine? 43. Determine the formula weight for the following: a. N2O5 b. CuSO4 C. Ca(HCO3)2 d. CaSO4 . 2 H2O 44. Wr ...
The Periodic Table
... Group 8-Nobel Gases • All the elements in group 8(except Helium) have 8 electrons in their outer shell. • This makes them stable and un-reactive. ...
... Group 8-Nobel Gases • All the elements in group 8(except Helium) have 8 electrons in their outer shell. • This makes them stable and un-reactive. ...
Final Exam review semester 1
... In science lab, your teacher gives you two small pieces of matter and tells you that one piece is a metal and one is a nonmetal. Without changing the size or shape of the pieces, how could you test them to determine which is the metal? ...
... In science lab, your teacher gives you two small pieces of matter and tells you that one piece is a metal and one is a nonmetal. Without changing the size or shape of the pieces, how could you test them to determine which is the metal? ...
Date_______________ Elements and facts of the periodic table
... _________________odorless, tasteless, colorless gas; lightest of all elements _______________natural material from which metal can be profitable extracted ________________most reactive metal _________________only metal liquid at room temperature ...
... _________________odorless, tasteless, colorless gas; lightest of all elements _______________natural material from which metal can be profitable extracted ________________most reactive metal _________________only metal liquid at room temperature ...
WILF 1 - GCSE Chemistry Help
... Group 7 Halogen elements and displacement reactions The reactivity of the Halogen elements decreases as you descend Group 7. Fluorine is too dangerous to try. In reactions between the other halogens and solutions of their potassium salts we can predict that the more reactive halogen, chlorine ...
... Group 7 Halogen elements and displacement reactions The reactivity of the Halogen elements decreases as you descend Group 7. Fluorine is too dangerous to try. In reactions between the other halogens and solutions of their potassium salts we can predict that the more reactive halogen, chlorine ...
ChemFinalgeocities
... When reacting with an atom of fluorine, an atom of lithium will lose an electron and become a lithium ...
... When reacting with an atom of fluorine, an atom of lithium will lose an electron and become a lithium ...
Chemistry 212 Name:
... 5. Discuss the halogens. (5 points) Each halogen is obtained by oxidation of the halide ion to the halogen in a molten salt, except fluorine. None of the halogens is particularly abundant in nature, however all are easily accessible in concentrated forms rendering this point moot. All halogens have ...
... 5. Discuss the halogens. (5 points) Each halogen is obtained by oxidation of the halide ion to the halogen in a molten salt, except fluorine. None of the halogens is particularly abundant in nature, however all are easily accessible in concentrated forms rendering this point moot. All halogens have ...
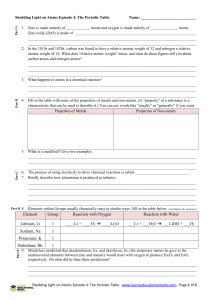
Element Group Reaction with Oxygen Reaction with Water Lithium
... 1. Zinc is made entirely of ____________ atoms and oxygen is made entirely of ______________ atoms. Zinc oxide (ZnO) is made of ______________________________________________________________ ______________________________________________________________________________________ ______________________ ...
... 1. Zinc is made entirely of ____________ atoms and oxygen is made entirely of ______________ atoms. Zinc oxide (ZnO) is made of ______________________________________________________________ ______________________________________________________________________________________ ______________________ ...
Chemistry Exam Review
... 3. Particles are always in constant random motion. 4. The particles in a substance attract each other. 5. The particles of a substance move faster when ...
... 3. Particles are always in constant random motion. 4. The particles in a substance attract each other. 5. The particles of a substance move faster when ...
Ch. 14 Test Review
... Ch. 14 Test Review CHEMICAL PERIODICITY COMPLETION decreases increases (3) groups (2) periods transition metals ionization energy atomic # noble gases representative electronegativity The periodic table organizes the elements into vertical ____________ and horizontal ____________ in order of increas ...
... Ch. 14 Test Review CHEMICAL PERIODICITY COMPLETION decreases increases (3) groups (2) periods transition metals ionization energy atomic # noble gases representative electronegativity The periodic table organizes the elements into vertical ____________ and horizontal ____________ in order of increas ...