CHAPTER 9 : CHEMICAL BONDING I
... (c) Li + N2 → Li3N (d) Al + S → Al2S3 9.20 For each of the following pairs of elements, state whether the binary compound they form is likely to be ionic or covalent. Write the empirical formula and name of the compound: (a) B and F, (b) K and Br. 9.26 Calculate the lattice energy of calcium chlorid ...
... (c) Li + N2 → Li3N (d) Al + S → Al2S3 9.20 For each of the following pairs of elements, state whether the binary compound they form is likely to be ionic or covalent. Write the empirical formula and name of the compound: (a) B and F, (b) K and Br. 9.26 Calculate the lattice energy of calcium chlorid ...
1 H NT Ch 12—Stoichiometry I. Review: Chemical Equations a
... ii. Carbon tetrachloride was prepared by reacting 100.0 g of carbon disulfide with 100.0 grams of chlorine gas. Calculate the theoretical and percent yield if 65.0 g of carbon tetrachloride was obtained. U ...
... ii. Carbon tetrachloride was prepared by reacting 100.0 g of carbon disulfide with 100.0 grams of chlorine gas. Calculate the theoretical and percent yield if 65.0 g of carbon tetrachloride was obtained. U ...
Brown, Le May, and Bursten: Chapter 2
... Some elements can form more than one compound when they react together (C & O: CO and CO2; N & O: N2O, NO, NO2, etc.). Dalton’s law predicted that the mass proportions should be proportional. Experiment confirmed this leading to this law. Law of multiple proportions: when two elements form more than ...
... Some elements can form more than one compound when they react together (C & O: CO and CO2; N & O: N2O, NO, NO2, etc.). Dalton’s law predicted that the mass proportions should be proportional. Experiment confirmed this leading to this law. Law of multiple proportions: when two elements form more than ...
H 2 and H 2 + O 2 g H 2 O and H 2 O Hydrogen + Oxygen g Water
... There are usually some obvious changes during a chemical reaction, including: A change in colour A gas coming off (you may see fizzing or bubbling) A change in temperature (the reaction mixture may get hotter) A solid may be formed when two solutions are mixed together ...
... There are usually some obvious changes during a chemical reaction, including: A change in colour A gas coming off (you may see fizzing or bubbling) A change in temperature (the reaction mixture may get hotter) A solid may be formed when two solutions are mixed together ...
Chemistry Nomenclature Notes
... Properties of ionic compounds: - solid at room temperature - dissolve in water (soluble) to form solution - solutions conduct electricity (electrolytes) - solutions can be any color - have higher melting & boiling points Charges must balance because one element gives up electrons and the other one a ...
... Properties of ionic compounds: - solid at room temperature - dissolve in water (soluble) to form solution - solutions conduct electricity (electrolytes) - solutions can be any color - have higher melting & boiling points Charges must balance because one element gives up electrons and the other one a ...
word-doc Practice for the final exam!
... 9. Precision refers to _______. a. how close a measured number is to other measured numbers b. how close a measured number is to the true value c. how close a measured number is to the calculated value d. how close a measured number is to zero e. how close a measured number is to infinity ...
... 9. Precision refers to _______. a. how close a measured number is to other measured numbers b. how close a measured number is to the true value c. how close a measured number is to the calculated value d. how close a measured number is to zero e. how close a measured number is to infinity ...
chapter 4 crossword pre-ap
... 4. The atomic radii of atoms ___ as you move left to right across a period. 5. Group 2 elements. 10. The number of valence electrons of a noble gas. 15. A reaction that affects the nucleus of an atom. 18. Aluminum has ___ valence electrons. 21. An electron that is found in the outermost shell of an ...
... 4. The atomic radii of atoms ___ as you move left to right across a period. 5. Group 2 elements. 10. The number of valence electrons of a noble gas. 15. A reaction that affects the nucleus of an atom. 18. Aluminum has ___ valence electrons. 21. An electron that is found in the outermost shell of an ...
Finals Review ans 2012sem 1
... Metallic bonding is similar to ionic bonding because there is an attraction between positively charged and negatively charged particles. ____ ...
... Metallic bonding is similar to ionic bonding because there is an attraction between positively charged and negatively charged particles. ____ ...
AP Chemistry Summer Assignment
... a. Kg b. Liter c. m3 d. mm e. kg/m3 f. Joule g. atm h. cal i. Torr j. g/ml 4. Most laboratory experiments are performed at room temperature at 65˚C. Express this temperature in: a. ˚F b. K 5. A cylinder rod formed from silicon is 46.0 cm long and has a mass of 3.00 kg. The density of silicon is 2.33 ...
... a. Kg b. Liter c. m3 d. mm e. kg/m3 f. Joule g. atm h. cal i. Torr j. g/ml 4. Most laboratory experiments are performed at room temperature at 65˚C. Express this temperature in: a. ˚F b. K 5. A cylinder rod formed from silicon is 46.0 cm long and has a mass of 3.00 kg. The density of silicon is 2.33 ...
Practice Test 1 (Chapters 1-7)
... Write you name and section number on both your test booklet and your Scantron Answer Sheet. Identify the choice that best completes the statement or answers the question and fill the space coresponding to your answer on your Scantron Answer Sheet. Be sure to erase mistakes ...
... Write you name and section number on both your test booklet and your Scantron Answer Sheet. Identify the choice that best completes the statement or answers the question and fill the space coresponding to your answer on your Scantron Answer Sheet. Be sure to erase mistakes ...
EXAM 1 - gozips.uakron.edu
... Calculate the percent by mass of lead in the mineral vanadinite, which has the formula Pb 5 (VO 4 ) 3 Cl. (A) 2.50 % (B) 10.79 % (C) 13.56 % (D) 57.14 % (E) 73.15 % ...
... Calculate the percent by mass of lead in the mineral vanadinite, which has the formula Pb 5 (VO 4 ) 3 Cl. (A) 2.50 % (B) 10.79 % (C) 13.56 % (D) 57.14 % (E) 73.15 % ...
AP Chemistry - School Webmasters
... structural formula, empirical formula, isotopes, cation, anion, and metalloid. 18. Determine number of protons and neutrons in each of the following. ...
... structural formula, empirical formula, isotopes, cation, anion, and metalloid. 18. Determine number of protons and neutrons in each of the following. ...
All you need to know about Additional Science
... If we have a solution containing 100 g of sodium hydroxide, how much chlorine gas should we pass through the solution to make bleach? Too much, and some chlorine will be wasted, too little and not all of the sodium hydroxide will react. ...
... If we have a solution containing 100 g of sodium hydroxide, how much chlorine gas should we pass through the solution to make bleach? Too much, and some chlorine will be wasted, too little and not all of the sodium hydroxide will react. ...
SCI 111
... Know how to read and use the Periodic Table of Elements (Fig 8.17 on page 237) Understand the “Key” ; Know basic aspects of these Families: ...
... Know how to read and use the Periodic Table of Elements (Fig 8.17 on page 237) Understand the “Key” ; Know basic aspects of these Families: ...
Limiting Reactant WS with Answers
... 8) The amino acid arginine is an essential component of all proteins. This compound contains 41.36% carbon, 8.10% hydrogen, and 32.17% nitrogen, with the remainder being oxygen. a) Determine the empirical formula of arginine. b) The molar mass of arginine is known to be between 100 and 200 g/mol. D ...
... 8) The amino acid arginine is an essential component of all proteins. This compound contains 41.36% carbon, 8.10% hydrogen, and 32.17% nitrogen, with the remainder being oxygen. a) Determine the empirical formula of arginine. b) The molar mass of arginine is known to be between 100 and 200 g/mol. D ...
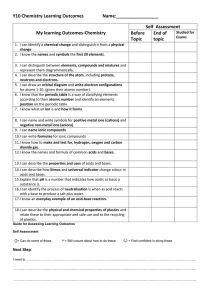
Y10 Chem SLOs 2016 File
... 4. I can describe the structure of the atom, including protons, neutrons and electrons. 5. I can draw an orbital diagram and write electron configurations for atoms 1-20. (given their atomic number). 6. I know that the periodic table is a way of classifying elements according to their atomic number ...
... 4. I can describe the structure of the atom, including protons, neutrons and electrons. 5. I can draw an orbital diagram and write electron configurations for atoms 1-20. (given their atomic number). 6. I know that the periodic table is a way of classifying elements according to their atomic number ...
Practice Exam 2 - Department of Chemistry and Biochemistry
... In a molecule with covalent bonding, A) atoms are held together by sharing electrons. B) oppositely charged ions are held together by strong electrical attractions. C) atoms of different metals form bonds. D) atoms of noble gases are held together by attractions between oppositely charged ions. E) a ...
... In a molecule with covalent bonding, A) atoms are held together by sharing electrons. B) oppositely charged ions are held together by strong electrical attractions. C) atoms of different metals form bonds. D) atoms of noble gases are held together by attractions between oppositely charged ions. E) a ...
compound - Coal City Unit #1
... • most symbols come from their names • some symbols come from Latin or Greek names • some elem. named in honor of person or place they were discovered • ea. elem. has its own unique set of chem. and physical props. ...
... • most symbols come from their names • some symbols come from Latin or Greek names • some elem. named in honor of person or place they were discovered • ea. elem. has its own unique set of chem. and physical props. ...
CHEMICAL REACTIONS Chapter 4
... 2. Find the number of atoms for each element on the left side. Compare those against the number of the atoms of the same element on the right side. 3. Determine where to place coefficients in front of formulas so that the left side has the same number of atoms as the right side for EACH element in o ...
... 2. Find the number of atoms for each element on the left side. Compare those against the number of the atoms of the same element on the right side. 3. Determine where to place coefficients in front of formulas so that the left side has the same number of atoms as the right side for EACH element in o ...
File
... G = given quantity of a reactant or product W = wanted quantity of a reactant or product A = number of moles of G in the balanced chemical equation B = number of moles of W in the balanced chemical equation EXAMPLE 1 (Mass-Mass): How many grams of copper (II) oxide are formed in the decomposition of ...
... G = given quantity of a reactant or product W = wanted quantity of a reactant or product A = number of moles of G in the balanced chemical equation B = number of moles of W in the balanced chemical equation EXAMPLE 1 (Mass-Mass): How many grams of copper (II) oxide are formed in the decomposition of ...
Elements Combine to Form Compounds
... a change in matter in which NEW substances are produced with NEW properties. Clues that May Indicate a Chemical Change ...
... a change in matter in which NEW substances are produced with NEW properties. Clues that May Indicate a Chemical Change ...
Naming Compounds
... a change in matter in which NEW substances are produced with NEW properties. Clues that May Indicate a Chemical Change ...
... a change in matter in which NEW substances are produced with NEW properties. Clues that May Indicate a Chemical Change ...
Unit 7: Chemical Equations & Reactions
... 1. Identify the most complex substance. 2. Beginning with that substance, choose an element that appears in only one reactant and one product. • Adjust the coefficients to obtain the same number of atoms of this element on both sides. • Balance polyatomic ions as a unit (if possible). • Re-write H2 ...
... 1. Identify the most complex substance. 2. Beginning with that substance, choose an element that appears in only one reactant and one product. • Adjust the coefficients to obtain the same number of atoms of this element on both sides. • Balance polyatomic ions as a unit (if possible). • Re-write H2 ...
PDF (Size: 41K)
... Explain, with reference to the standard electrode potential for sodium and hydrogen, why sodium is manufactured using this method rather than by the electrolysis of aqueous sodium chloride. Na+(aq) + e– ...
... Explain, with reference to the standard electrode potential for sodium and hydrogen, why sodium is manufactured using this method rather than by the electrolysis of aqueous sodium chloride. Na+(aq) + e– ...