ELEMENTS and THEIR PROPERTIES
... PROPERTIES OF NONMETALS- usually gases or brittle solids at room temperature; are not malleable or ductile; usually poor conductors of heat and electricity; usually not lustrous Ionic compounds- form when nonmetals gain electrons from metals and become negative ions Covalent compounds- form when non ...
... PROPERTIES OF NONMETALS- usually gases or brittle solids at room temperature; are not malleable or ductile; usually poor conductors of heat and electricity; usually not lustrous Ionic compounds- form when nonmetals gain electrons from metals and become negative ions Covalent compounds- form when non ...
Activity 16 Elements and the Periodic Table
... Do you see another of the patterns that Mendeleev and other scientists recognized? ___________ Neutral atoms contain equal numbers of protons (+) and electrons (-). Because of this, you know that neutral atoms of lithium, beryllium, boron and carbon also have 3, 4, 5, and 6 electrons respectively. W ...
... Do you see another of the patterns that Mendeleev and other scientists recognized? ___________ Neutral atoms contain equal numbers of protons (+) and electrons (-). Because of this, you know that neutral atoms of lithium, beryllium, boron and carbon also have 3, 4, 5, and 6 electrons respectively. W ...
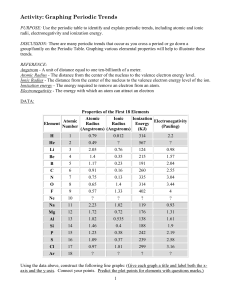
LSHS Graphing Periodic Trends Lab
... 8. Going across a period from left to right: The [ p+, N , e- ] in the nucleus increase, thus pulling the [ p+ , N , e- ] closer towards the center of the atom and [ increasing, decreasing ] the atomic radii. Because of this increase in electromagnetic, strong force atoms tend to [ gain, lose] elect ...
... 8. Going across a period from left to right: The [ p+, N , e- ] in the nucleus increase, thus pulling the [ p+ , N , e- ] closer towards the center of the atom and [ increasing, decreasing ] the atomic radii. Because of this increase in electromagnetic, strong force atoms tend to [ gain, lose] elect ...
Dalton`s Laws worksheet
... Dalton’s Atomic Theory of Matter 1. Which of the following statements is part of Dalton’s atomic theory of matter? a. All atoms are identical b. All atoms of a given element are identical c. All atoms differ from one another d. Atoms of the same element can have a different shape 2. Dalton suggested ...
... Dalton’s Atomic Theory of Matter 1. Which of the following statements is part of Dalton’s atomic theory of matter? a. All atoms are identical b. All atoms of a given element are identical c. All atoms differ from one another d. Atoms of the same element can have a different shape 2. Dalton suggested ...
In-Class Exam - Fayetteville State University
... 14. Isotopes are atoms that have the same number of ______ but differing number of ______. A) neutrons, protons B) protons, electrons C) neutrons, electrons D) electrons, protons ...
... 14. Isotopes are atoms that have the same number of ______ but differing number of ______. A) neutrons, protons B) protons, electrons C) neutrons, electrons D) electrons, protons ...
Atoms, Bonding, and the Periodic Table Electron Dot Diagrams
... The valence electrons of an atom are shown as dots around the symbol of the element. Complete the electron dot diagram for neon. ...
... The valence electrons of an atom are shown as dots around the symbol of the element. Complete the electron dot diagram for neon. ...
temperature change energy change
... b. exothermic c. a decomposition d. endothermic 3. When an exothermic reaction takes place, the reaction mixture gets hotter because? a. exothermic changes always produce gases b. more energy is released when new bonds are made than is needed to break existing bonds c. in an exothermic change the pr ...
... b. exothermic c. a decomposition d. endothermic 3. When an exothermic reaction takes place, the reaction mixture gets hotter because? a. exothermic changes always produce gases b. more energy is released when new bonds are made than is needed to break existing bonds c. in an exothermic change the pr ...
File - Unit #1-0
... 18. What sublevels are filling across the Transition Elements? 19. Elements within a group have a similar number of 20. Elements across a series have the same number of 21. A colored ion generally indicates a 22. As you go down a group, the elements generally become ( more / less) metallic. 23. The ...
... 18. What sublevels are filling across the Transition Elements? 19. Elements within a group have a similar number of 20. Elements across a series have the same number of 21. A colored ion generally indicates a 22. As you go down a group, the elements generally become ( more / less) metallic. 23. The ...
Atomic Theories and Models - MrD-Home
... The chemical equation for the reaction of methane and oxygen is ______ yet properly balanced because the atoms of the elements on the product side do not ______ the atoms of each element on the reactant side of the equation. The _________________________, which states that matter can neither be ____ ...
... The chemical equation for the reaction of methane and oxygen is ______ yet properly balanced because the atoms of the elements on the product side do not ______ the atoms of each element on the reactant side of the equation. The _________________________, which states that matter can neither be ____ ...
qp13 - Smart Edu Hub
... Which row correctly shows the physical state of element X at room temperature and its reactivity compared with that of iodine? physical state of element X at room temperature ...
... Which row correctly shows the physical state of element X at room temperature and its reactivity compared with that of iodine? physical state of element X at room temperature ...
NAME…………… - Kcse Online
... ____________________________________________________________________________ ____________________________________________________________________________ ____________________________________________________________________________ _____________________________________________________________________ ...
... ____________________________________________________________________________ ____________________________________________________________________________ ____________________________________________________________________________ _____________________________________________________________________ ...
Stoichiometry - Cloudfront.net
... 7. A 105.5 mg sample of a white substance is suspected to be cocaine, C 17H21NO4. The substance formed 279.3 mg of CO2 and 66.46 mg H2O on combustion. The compound contains 4.680% N by mass. Is the white solid cocaine? 8. An unknown compound (molar mass = 176 g/mol) contains 68.2 mass % C, 6.86 mass ...
... 7. A 105.5 mg sample of a white substance is suspected to be cocaine, C 17H21NO4. The substance formed 279.3 mg of CO2 and 66.46 mg H2O on combustion. The compound contains 4.680% N by mass. Is the white solid cocaine? 8. An unknown compound (molar mass = 176 g/mol) contains 68.2 mass % C, 6.86 mass ...
Chapter 2 - Chemical Context of Life
... Ionic bonds occur when two atoms are so unequal in their attraction for e- that one atom will strip the e- from its partner. These ...
... Ionic bonds occur when two atoms are so unequal in their attraction for e- that one atom will strip the e- from its partner. These ...
Chapter 22 Chemistry of The NonMetals
... Xenon fluorides have Xe in the +2 to +8 oxidation states. Noble gas compounds violate the octet rule. In the presence of water, xenon fluorides form oxyfluorides: • XeF6(s) + H2O(l) → XeOF4(l) + 2HF • XeF6(s) + 3H2O(l) → XeO3(aq) + 6HF The only other noble gas compound known is KrF2, which decompose ...
... Xenon fluorides have Xe in the +2 to +8 oxidation states. Noble gas compounds violate the octet rule. In the presence of water, xenon fluorides form oxyfluorides: • XeF6(s) + H2O(l) → XeOF4(l) + 2HF • XeF6(s) + 3H2O(l) → XeO3(aq) + 6HF The only other noble gas compound known is KrF2, which decompose ...
The Periodic Table & Formation of Ions
... Increases from left to right across a period Decreases from top to bottom in a group ...
... Increases from left to right across a period Decreases from top to bottom in a group ...
chapter2 - AlvarezHChem
... 1. An element is composed of tiny particles called atoms 2. All atoms of the same element have the same chemical properties 3. In an ordinary chemical reaction, atoms rearrange their bonds but atoms are not created or destroyed 4. Compounds are formed when two or more atoms of different element comb ...
... 1. An element is composed of tiny particles called atoms 2. All atoms of the same element have the same chemical properties 3. In an ordinary chemical reaction, atoms rearrange their bonds but atoms are not created or destroyed 4. Compounds are formed when two or more atoms of different element comb ...
BITSAT Chemistry
... constant pH of 9, the volume of 5 M KCN solution required to be together with water the boiled together with water, the boiling added to 10 ml of 2 M HCN solution is a ...
... constant pH of 9, the volume of 5 M KCN solution required to be together with water the boiled together with water, the boiling added to 10 ml of 2 M HCN solution is a ...
Atoms, Ions, and Molecules File
... Atoms of different elements are different. • Atoms are not changed into different atoms in a chemical reaction. • Compounds are formed when atoms of two or more elements combine. ...
... Atoms of different elements are different. • Atoms are not changed into different atoms in a chemical reaction. • Compounds are formed when atoms of two or more elements combine. ...
File
... • These seven elements will never be alone. If only the element is there, you must put a 2 subscript. • It is still possible for there to be only one of these elements, if it is bonded to something else. ▫ Example: MgO ...
... • These seven elements will never be alone. If only the element is there, you must put a 2 subscript. • It is still possible for there to be only one of these elements, if it is bonded to something else. ▫ Example: MgO ...
Ways the Periodic Table is Organized
... Ways the Periodic Table is Organized Use the Chemical Interactions textbook to describe the following ways the periodic table is organized. Be sure to give examples as well as the definition: A: Groups (p. 22) B: Periods (p. 22) C: Reactivity (p. 26) ...
... Ways the Periodic Table is Organized Use the Chemical Interactions textbook to describe the following ways the periodic table is organized. Be sure to give examples as well as the definition: A: Groups (p. 22) B: Periods (p. 22) C: Reactivity (p. 26) ...
Chemistry IGCSE Revision PDF File
... If a metal is more reactive than hydrogen its ions stay in solution and hydrogen bubbles off ...
... If a metal is more reactive than hydrogen its ions stay in solution and hydrogen bubbles off ...
Chemistry
... a. aqueous barium chloride reacts with aqueous silver sulfate to form solid silver chloride and solid barium sulfate b. calcium carbonate when heated forms calcium oxide and carbon dioxide ...
... a. aqueous barium chloride reacts with aqueous silver sulfate to form solid silver chloride and solid barium sulfate b. calcium carbonate when heated forms calcium oxide and carbon dioxide ...
Atomic and Molecular Structure – Standard 1 Review
... 1b.1 On the Periodic Table, be able to identify Metals, Nonmetals, and Metalloids (Semi-metals). 1c.1-1c.3 On the Periodic Table, be able to identify Alkali Metals, Alkaline Earth Metals, Transition Metals, Chalcogens, Halogens, and Noble Gases. 1c.4 – 1c.5 On the Periodic Table, be able to identify ...
... 1b.1 On the Periodic Table, be able to identify Metals, Nonmetals, and Metalloids (Semi-metals). 1c.1-1c.3 On the Periodic Table, be able to identify Alkali Metals, Alkaline Earth Metals, Transition Metals, Chalcogens, Halogens, and Noble Gases. 1c.4 – 1c.5 On the Periodic Table, be able to identify ...