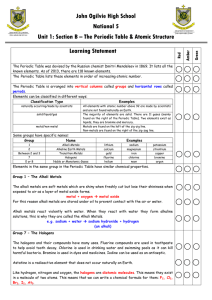
Chemistry Semester One Exam Review Name:
... d) Double replacement e) Single replacement 17. Complete the word equation, write and balance the equation using symbols and indicate the type of the reaction on the left. a. Propane (C3H8) burns in air b. Magnesium chloride + silver nitrate c. Zn reacts with hydrochloric acid d. Nitrogen gas ...
... d) Double replacement e) Single replacement 17. Complete the word equation, write and balance the equation using symbols and indicate the type of the reaction on the left. a. Propane (C3H8) burns in air b. Magnesium chloride + silver nitrate c. Zn reacts with hydrochloric acid d. Nitrogen gas ...
Midterm Review (2014-2015) - Questions 1. What is matter? Provide
... 4. Compare the properties of a solid, a liquid, and a gas. 5. Copper has reddish-brown metal that is easily stretched to make wires. Over a period of time, it will react in air transforming it into the greenish copper (II) oxide. This is observed on ...
... 4. Compare the properties of a solid, a liquid, and a gas. 5. Copper has reddish-brown metal that is easily stretched to make wires. Over a period of time, it will react in air transforming it into the greenish copper (II) oxide. This is observed on ...
Periodic Table and Atomic Structure Summary
... The alkali metals are soft metals which are shiny when freshly cut but lose their shininess when exposed to air as a layer of metal oxide forms. metal + oxygen metal oxide For this reason alkali metals are stored under oil to prevent contact with the air or water. Alkali metals react violently wit ...
... The alkali metals are soft metals which are shiny when freshly cut but lose their shininess when exposed to air as a layer of metal oxide forms. metal + oxygen metal oxide For this reason alkali metals are stored under oil to prevent contact with the air or water. Alkali metals react violently wit ...
Targets of Opportunity
... 10,000 people died in the three days after the explosion and 15,000 more have died since. ...
... 10,000 people died in the three days after the explosion and 15,000 more have died since. ...
Exam on Matter through Bonding
... 9. Which terms are used to identify pure substances? (1) an element and a mixture (2) an element and a compound (3) a solution and a mixture (4) a solution and a compound 10. The bonds in the compound MgSO4 can be described as ...
... 9. Which terms are used to identify pure substances? (1) an element and a mixture (2) an element and a compound (3) a solution and a mixture (4) a solution and a compound 10. The bonds in the compound MgSO4 can be described as ...
NAME
... b) aluminum bromide solution and chlorine gas react to form aluminum chloride and bromine gas. ...
... b) aluminum bromide solution and chlorine gas react to form aluminum chloride and bromine gas. ...
Chapter 2 - Speedway High School
... • An element is a substance that cannot be broken down to other substances by chemical reactions • A compound is a substance consisting of two or more elements in a fixed ratio ...
... • An element is a substance that cannot be broken down to other substances by chemical reactions • A compound is a substance consisting of two or more elements in a fixed ratio ...
The Periodic Table of Elements
... therefore it becomes more difficult for the nucleus to attract an electron to form an ion most reactive is fluorine; least reactive is iodine all halogens form ions with single negative charge eg F-, Cl-, BrExists as diatomic molecules eg F2, Cl2, Br2, I2 reacts vigorously with metals to form ioni ...
... therefore it becomes more difficult for the nucleus to attract an electron to form an ion most reactive is fluorine; least reactive is iodine all halogens form ions with single negative charge eg F-, Cl-, BrExists as diatomic molecules eg F2, Cl2, Br2, I2 reacts vigorously with metals to form ioni ...
UNIT 1 - MATTER AND CHEMICAL BONDING
... f) ferrous iodide l) cobalt(III) sulphate 5. Classify each of the following reactions as synthesis, single displacement, double displacement, combustion or decomposition. a) iron + copper(I) nitrate iron(II) nitrate + copper b) phosphorus + oxygen diphosphorus pentoxide c) calcium carbonate ca ...
... f) ferrous iodide l) cobalt(III) sulphate 5. Classify each of the following reactions as synthesis, single displacement, double displacement, combustion or decomposition. a) iron + copper(I) nitrate iron(II) nitrate + copper b) phosphorus + oxygen diphosphorus pentoxide c) calcium carbonate ca ...
The Periodic Table Notes
... Known as halogens because they react with metals to create salts At room temperature, fluorine and chlorine are gases, bromine is a liquid, and iodine is a solid ...
... Known as halogens because they react with metals to create salts At room temperature, fluorine and chlorine are gases, bromine is a liquid, and iodine is a solid ...
Periodic Table Puzzle
... The code letters A to Z have been assigned to represent the first 26 representative elements in the Periodic Table. The letters do not relate to the actual chemical symbols for these elements. Your challenge is to put the code letters in the correct boxes in the Periodic Table, based on the ...
... The code letters A to Z have been assigned to represent the first 26 representative elements in the Periodic Table. The letters do not relate to the actual chemical symbols for these elements. Your challenge is to put the code letters in the correct boxes in the Periodic Table, based on the ...
CHM 212 - The Federal University of Agriculture, Abeokuta
... As stated earlier,HCl and also HBR and HI behave as weak acids in acetic acid. But the extent of their ionization varies as follows; HI>HBr>HCl. These are acids are normally classified as strong acids in aqueous solution because they are fully ionized. But acetic acid gives a contrast to this, thus ...
... As stated earlier,HCl and also HBR and HI behave as weak acids in acetic acid. But the extent of their ionization varies as follows; HI>HBr>HCl. These are acids are normally classified as strong acids in aqueous solution because they are fully ionized. But acetic acid gives a contrast to this, thus ...
Units 3 and 4 Revision
... Standard Grade Revision Units 3 and 4 Q1. The box below shows the names of some elements. ...
... Standard Grade Revision Units 3 and 4 Q1. The box below shows the names of some elements. ...
Topic 3&4 Atoms and the per.table
... Standard Grade Revision Units 3 and 4 Q1. The box below shows the names of some elements. ...
... Standard Grade Revision Units 3 and 4 Q1. The box below shows the names of some elements. ...
How Atoms Differ (Section 4.3) part 1
... nuclear charge, so the atom is neutral? • So how many electrons • Atomic number = number of protons = number of electrons • How many electons are in an atom of: – Hydrogen? – Lead? – Chlorine? ...
... nuclear charge, so the atom is neutral? • So how many electrons • Atomic number = number of protons = number of electrons • How many electons are in an atom of: – Hydrogen? – Lead? – Chlorine? ...
CHEMISTRY
... Held together by bonds Covalent bonds (strong): 2 or more atoms share electrons Ionic Bonds (weak): attractions between + and ions ...
... Held together by bonds Covalent bonds (strong): 2 or more atoms share electrons Ionic Bonds (weak): attractions between + and ions ...
File
... halogens? All halogens have seven electrons in their outer shell. This means that: They can easily obtain a full outer shell by gaining one electron. They all gain an electron in reactions to form negative ions with a -1 charge. ...
... halogens? All halogens have seven electrons in their outer shell. This means that: They can easily obtain a full outer shell by gaining one electron. They all gain an electron in reactions to form negative ions with a -1 charge. ...
Group 17: The Halogens - Chemwiki
... react with the noble gas xenon and form the strong oxidizing agent Xenon Difluoride (XeF2). There are many uses for fluorine, which will be discussed in Part VI of this article. 2. Chlorine - Chlorine has the atomic number 17 and the chemical symbol Cl. Chlorine was discovered in 1774 by extracting ...
... react with the noble gas xenon and form the strong oxidizing agent Xenon Difluoride (XeF2). There are many uses for fluorine, which will be discussed in Part VI of this article. 2. Chlorine - Chlorine has the atomic number 17 and the chemical symbol Cl. Chlorine was discovered in 1774 by extracting ...
Equation Intro Worksheet 1213
... Look at the above picture and the ones on pages 325-327 to see why these reactions are drawn the way they are…(note that the book uses colors to identify each element’s atoms where I’ve used letters because this is a black and white photocopy) 5. In the space below, draw the reaction written…use num ...
... Look at the above picture and the ones on pages 325-327 to see why these reactions are drawn the way they are…(note that the book uses colors to identify each element’s atoms where I’ve used letters because this is a black and white photocopy) 5. In the space below, draw the reaction written…use num ...
Name________________ Hour____ Chapter 11 Review 1. Name
... j. How many total atoms are reacting? 6 d. Name the element in the reaction. Oxygen k. How many total atoms are produced? 6 e. Name the compound on the reactant side. Carbon monoxide l. Which substances have double/triple g. List all of the subscripts in the reaction 2,2 bonds? All have double/tripl ...
... j. How many total atoms are reacting? 6 d. Name the element in the reaction. Oxygen k. How many total atoms are produced? 6 e. Name the compound on the reactant side. Carbon monoxide l. Which substances have double/triple g. List all of the subscripts in the reaction 2,2 bonds? All have double/tripl ...
Me, Myself, I, Chlorine BY: Ethan. BP:2
... Me, Myself, I, Chlorine BY: Ethan. B P:2 It started in a lab in Sweden. Chlorine was first produced by Carl Wilhelm Scheele, a Swedish chemist, when he combined the mineral pyrolusite (MnO2) with hydrochloric acid (HCl) in 1774. That is how i was born. (Beautiful right?) MY ...
... Me, Myself, I, Chlorine BY: Ethan. B P:2 It started in a lab in Sweden. Chlorine was first produced by Carl Wilhelm Scheele, a Swedish chemist, when he combined the mineral pyrolusite (MnO2) with hydrochloric acid (HCl) in 1774. That is how i was born. (Beautiful right?) MY ...
Worksheet 2: 1-19-17 - Iowa State University
... 9. In an experiment, a scientist prepared 3 different compounds containing only iodine and fluorine. Calculate the mass of iodine per fluorine in each compound. Compound Mass Iodine (g) Mass Fluorine (g) ...
... 9. In an experiment, a scientist prepared 3 different compounds containing only iodine and fluorine. Calculate the mass of iodine per fluorine in each compound. Compound Mass Iodine (g) Mass Fluorine (g) ...