Anticipation Guide for Chem Talk pg 370-373
... 2. Dalton predicted the elements hydrogen and oxygen combine to form water ...
... 2. Dalton predicted the elements hydrogen and oxygen combine to form water ...
Practice Test 11 - U of L Class Index
... A chunk of white phosphorus weighing 6.58 grams is put in a 750 mL flask containing dry argon (which is then removed using a vacuum, leaving only the phosphorus in the flask). A separate 750 mL flask contains 3.15 bar of fluorine gas (at 19.65 °C). The two flasks are connected so that the two compou ...
... A chunk of white phosphorus weighing 6.58 grams is put in a 750 mL flask containing dry argon (which is then removed using a vacuum, leaving only the phosphorus in the flask). A separate 750 mL flask contains 3.15 bar of fluorine gas (at 19.65 °C). The two flasks are connected so that the two compou ...
ATOMS ELEMENTS PERIODIC TABLE MOLECULES COMPOUNDS
... • What is the difference between a compound and a molecule? • A molecule is formed when two or more atoms join together chemically. A compound is a molecule that contains at least two different elements. All compounds are molecules but not all molecules are compounds. • Molecular hydrogen (H2), mole ...
... • What is the difference between a compound and a molecule? • A molecule is formed when two or more atoms join together chemically. A compound is a molecule that contains at least two different elements. All compounds are molecules but not all molecules are compounds. • Molecular hydrogen (H2), mole ...
Chemical Reactions Practice Test
... b) the same number of atoms c) half the number of atoms _____2. What unit allows chemists to compare one chemical to another? a) amu b) kilograms c) atomic weights d) moles ______3. The coefficients from the balanced equation represent a) mole ratios b) masses c) molar masses _______4. In a chemical ...
... b) the same number of atoms c) half the number of atoms _____2. What unit allows chemists to compare one chemical to another? a) amu b) kilograms c) atomic weights d) moles ______3. The coefficients from the balanced equation represent a) mole ratios b) masses c) molar masses _______4. In a chemical ...
Test #5 Review
... Which force holds the nucleus together? the strong force Which force holds the electrons around the nucleus? the electromagnetic force Define mass number. number of protons + number of neutrons ...
... Which force holds the nucleus together? the strong force Which force holds the electrons around the nucleus? the electromagnetic force Define mass number. number of protons + number of neutrons ...
Chemistry Module 1- Basic Revision Notes 1.1a Atomic Structure 1.1
... These molecules are very common in GCSE symbol equations. Many of them tend to be gases. The following list of diatomic molecules should be known and learned:- ...
... These molecules are very common in GCSE symbol equations. Many of them tend to be gases. The following list of diatomic molecules should be known and learned:- ...
Midterm Review
... • A strontium atom differs from a strontium ion in that the atom has a greater 1. number of electrons 2 number of protons ...
... • A strontium atom differs from a strontium ion in that the atom has a greater 1. number of electrons 2 number of protons ...
for the quiz on 6 mar
... 19.43 Of the group 3A elements, boron is the only one on the “nonmetals” side of the line. The rest of the group 3A elements participate in ionic bonding, and form ions. Boron, on the other hand, forms compounds through sharing of electrons in covalent bonds. 19.98 In order to have a stronger acid, ...
... 19.43 Of the group 3A elements, boron is the only one on the “nonmetals” side of the line. The rest of the group 3A elements participate in ionic bonding, and form ions. Boron, on the other hand, forms compounds through sharing of electrons in covalent bonds. 19.98 In order to have a stronger acid, ...
gr11chemreview
... The following questions highlight the main knowledge and skills from grade 11 chemistry. A good understanding of the concepts covered in grade 11 chemistry is essential for success in grade 12 chemistry and you may need to do some independent review of some material if you do not have a clear unders ...
... The following questions highlight the main knowledge and skills from grade 11 chemistry. A good understanding of the concepts covered in grade 11 chemistry is essential for success in grade 12 chemistry and you may need to do some independent review of some material if you do not have a clear unders ...
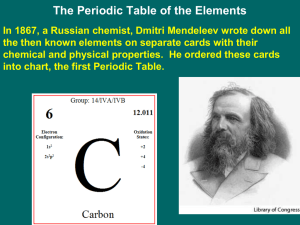
2.2 Periodic Chart
... earth elements also react with water and are very common elements in earth rocks (calcium and magnesium especially). ...
... earth elements also react with water and are very common elements in earth rocks (calcium and magnesium especially). ...
Cl Cl and
... 14. If a pair of electrons shared between two atoms is called a covalent bond, how many covalent bonds exist between the two chlorine atoms in Cl2(g)? One covalent bond ...
... 14. If a pair of electrons shared between two atoms is called a covalent bond, how many covalent bonds exist between the two chlorine atoms in Cl2(g)? One covalent bond ...
inorganic chemistry
... extremely dry glass, or metals such as copper or steel which form a protective layer of fluoride on their surface. The high reactivity of fluorine means that once it does react with something, it bonds with it so strongly that the resulting molecule is very inert and non-reactive to anything else. F ...
... extremely dry glass, or metals such as copper or steel which form a protective layer of fluoride on their surface. The high reactivity of fluorine means that once it does react with something, it bonds with it so strongly that the resulting molecule is very inert and non-reactive to anything else. F ...
Mongar Higher Secondary School
... In the above reaction, the volume of hydrogen that will combine with 30 litres of oxygen to form steam is a) 60 litres b) 50 litres c) 40 litres ...
... In the above reaction, the volume of hydrogen that will combine with 30 litres of oxygen to form steam is a) 60 litres b) 50 litres c) 40 litres ...
REVIEW TEST 4.5 weeks
... A Material Safety Data Sheet (MSDS) is a document that contains information on the potential health effects of exposure to chemicals, or other potentially dangerous substances, and on safe working procedures when handling chemical products. ...
... A Material Safety Data Sheet (MSDS) is a document that contains information on the potential health effects of exposure to chemicals, or other potentially dangerous substances, and on safe working procedures when handling chemical products. ...
Are You suprised ?
... a. two elements in the same group (A) b. two elements in the same period (B) c. a transition metal (C) d. A lanthanide (D) e. An alkali Earth Metal (E) f. A non-metal (F) g. A metalloid (G) ...
... a. two elements in the same group (A) b. two elements in the same period (B) c. a transition metal (C) d. A lanthanide (D) e. An alkali Earth Metal (E) f. A non-metal (F) g. A metalloid (G) ...
Chapter 12 The Periodic Table
... silvery in their pure form and are highly reactive. This group includes the elements lithium (Li), sodium (Na), and potassium (K). ...
... silvery in their pure form and are highly reactive. This group includes the elements lithium (Li), sodium (Na), and potassium (K). ...
snc 2do unit: chemistry unit test review questions
... 2. Write the formulas for the following compounds. A) sulphur trioxide B) tin (IV) bromide C) iron (II) oxide D) carbon monoxide E) rubidium oxide F) nitric acid G) copper (II) sulphide H) aluminum sulphate I) silicon dioxide J) phosphorus pentachloride 3. What do the elements in the same group all ...
... 2. Write the formulas for the following compounds. A) sulphur trioxide B) tin (IV) bromide C) iron (II) oxide D) carbon monoxide E) rubidium oxide F) nitric acid G) copper (II) sulphide H) aluminum sulphate I) silicon dioxide J) phosphorus pentachloride 3. What do the elements in the same group all ...
Answers
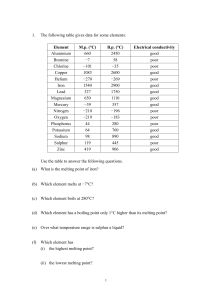
... (a) What is the melting point of iron? (b) Which element melts at −7°C? (c) Which element boils at 280°C? (d) Which element has a boiling point only 1°C higher than its melting point? ...
... (a) What is the melting point of iron? (b) Which element melts at −7°C? (c) Which element boils at 280°C? (d) Which element has a boiling point only 1°C higher than its melting point? ...
Conservation of Mass Lab
... during a chemical reaction. This means that all chemical reactions must be balanced—the number of atoms, moles, and ultimately the total mass must be conserved during a chemical process. Here are the rules to follow when balancing equations: ...
... during a chemical reaction. This means that all chemical reactions must be balanced—the number of atoms, moles, and ultimately the total mass must be conserved during a chemical process. Here are the rules to follow when balancing equations: ...
Oxidation Number Rules
... 3. The oxidation numbers of some common atoms are: a. Fluorine, the most electronegative element, is -1 in all fluorine containing compounds. b. In most oxygen containing compounds oxygen is -2. In peroxides (i.e. H2O2) each oxygen has an oxidation number of -1. In the compound OF2, the oxygen atom ...
... 3. The oxidation numbers of some common atoms are: a. Fluorine, the most electronegative element, is -1 in all fluorine containing compounds. b. In most oxygen containing compounds oxygen is -2. In peroxides (i.e. H2O2) each oxygen has an oxidation number of -1. In the compound OF2, the oxygen atom ...
Chem 1100 Chapter Three Study Guide Outline I. Molar Mass and
... 20. Sodium metal and water react to form hydrogen and sodium hydroxide. If 5.98 g of sodium react with water to form 0.26 g of hydrogen and 10.40 g of sodium hydroxide, what mass of water was consumed in the reaction? a. 10.66 g b. 4.68 g c. 10.14 g d. 5.98 g 21. What is the chemical formula for str ...
... 20. Sodium metal and water react to form hydrogen and sodium hydroxide. If 5.98 g of sodium react with water to form 0.26 g of hydrogen and 10.40 g of sodium hydroxide, what mass of water was consumed in the reaction? a. 10.66 g b. 4.68 g c. 10.14 g d. 5.98 g 21. What is the chemical formula for str ...
AS Paper 1 Practice Paper 4 - A
... A vinegar solution was obtained from a leading supplier. The label gave the concentration of ethanoic acid as 56.1 gdm-3. A student was asked to check the concentration of the acid using standard sodium hydroxide solution. The student decided to use a measuring cylinder to obtain 25.0 cm3 of the sup ...
... A vinegar solution was obtained from a leading supplier. The label gave the concentration of ethanoic acid as 56.1 gdm-3. A student was asked to check the concentration of the acid using standard sodium hydroxide solution. The student decided to use a measuring cylinder to obtain 25.0 cm3 of the sup ...
Grade 11 Chemistry E.. - hrsbstaff.ednet.ns.ca
... g. Na2SO4(aq) + BaCl2(aq) → BaSO4(s) + NaCl(aq) h. CH3OH(l) + O2(g) → CO2(g) + H2O(g) 25. Classify each of the above according to the 5 types of reactions (composition, decomposition, single replacement, double replacement and combustion). 26. Write the formula for each material correctly and then b ...
... g. Na2SO4(aq) + BaCl2(aq) → BaSO4(s) + NaCl(aq) h. CH3OH(l) + O2(g) → CO2(g) + H2O(g) 25. Classify each of the above according to the 5 types of reactions (composition, decomposition, single replacement, double replacement and combustion). 26. Write the formula for each material correctly and then b ...