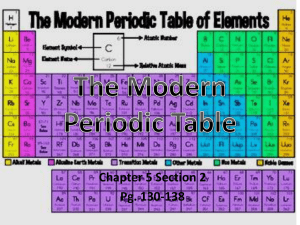
How are properties of atoms used to organize elements into the
... Elements are arranged in the periodic table according to their atomic structure and properties • PERIODS – rows on the periodic table (side ways) • represents the number of energy levels that contain electrons • FAMILES – columns or groups on the periodic table (up and down) • represents the number ...
... Elements are arranged in the periodic table according to their atomic structure and properties • PERIODS – rows on the periodic table (side ways) • represents the number of energy levels that contain electrons • FAMILES – columns or groups on the periodic table (up and down) • represents the number ...
CLASSIFICATION OF THE ELEMENTS
... ________ 12. Removing one electron from an atom results in the formation of a positive ion with a 11 charge. ________ 13. The relative radii of atoms are estimated as being half the distance between nuclei in diatomic molecules. ________ 14. Atoms with high electronegativity tend to form positive io ...
... ________ 12. Removing one electron from an atom results in the formation of a positive ion with a 11 charge. ________ 13. The relative radii of atoms are estimated as being half the distance between nuclei in diatomic molecules. ________ 14. Atoms with high electronegativity tend to form positive io ...
Chapter 4: Energy Guided Reading
... Chapter 17: Properties of Atoms and the Periodic Table- Key Read the indicated sections and answer the following questions. They are IN ORDER from the text. Page 506-511 1. What are the particles that make up the atom and where are they located? Particles that make up atoms include protons and neutr ...
... Chapter 17: Properties of Atoms and the Periodic Table- Key Read the indicated sections and answer the following questions. They are IN ORDER from the text. Page 506-511 1. What are the particles that make up the atom and where are they located? Particles that make up atoms include protons and neutr ...
20161025140773
... • Because the scale repeats at regular eightnote intervals, the scale is an example of a periodic pattern • The sounds of musical notes that are separated by an octave are related, but they are not identical- similar to elements in columns ...
... • Because the scale repeats at regular eightnote intervals, the scale is an example of a periodic pattern • The sounds of musical notes that are separated by an octave are related, but they are not identical- similar to elements in columns ...
CHEMISTRY NOTES 9.1.1 ATOMS, ELEMENTS, PERIODIC TABLE
... Malleable (can be shaped), fusible (can be fused or melted), ductile (can be formed into wire) Lose the electrons in their outer shell; form metallic and ionic bonds; transmit heat & electricity easily because of flow of electrons; form metallic bonds between atoms of the same element All metals are ...
... Malleable (can be shaped), fusible (can be fused or melted), ductile (can be formed into wire) Lose the electrons in their outer shell; form metallic and ionic bonds; transmit heat & electricity easily because of flow of electrons; form metallic bonds between atoms of the same element All metals are ...
ReviewCat1 - greenslime.info
... Conductivity - ability to transfer heat and/or electricity Density - measure of a materials mass per unit volume (m/v) Luster - describes how a material reflect light Malleability - materials flexibility without breaking Metals - good conductors of heat/electricity Metalloids - have properties of bo ...
... Conductivity - ability to transfer heat and/or electricity Density - measure of a materials mass per unit volume (m/v) Luster - describes how a material reflect light Malleability - materials flexibility without breaking Metals - good conductors of heat/electricity Metalloids - have properties of bo ...
College Chemistry – Atomic Structure / Periodic Table Test Study
... Know quantum theory: wave properties of electrons, probable location, quantum #'s Know what an isotope is and be able to determine its number of neutrons Know the shapes of electron orbitals s, p, and d Be able to write electron configurations in "longhand", "shorthand" (with noble gas notation), an ...
... Know quantum theory: wave properties of electrons, probable location, quantum #'s Know what an isotope is and be able to determine its number of neutrons Know the shapes of electron orbitals s, p, and d Be able to write electron configurations in "longhand", "shorthand" (with noble gas notation), an ...
L2: The Atom, Standard Notation and Borh
... Therefore the number of neutrons is? ________________________________ Question 1: In standard notation nitrogen would be written as How many protons, electrons and neutrons does nitrogen have? #p+ = #e-= #no= ...
... Therefore the number of neutrons is? ________________________________ Question 1: In standard notation nitrogen would be written as How many protons, electrons and neutrons does nitrogen have? #p+ = #e-= #no= ...
Name________________________ Period____ Date
... 1. The horizontal rows on the periodic table are called periods_. 2. The vertical columns on the periodic table are called groups or families. 3. The Periodic Table is arranged by increasing atomic number. __. 4. Describe the location of metals, nonmetals, and metalloids on the periodic table. Metal ...
... 1. The horizontal rows on the periodic table are called periods_. 2. The vertical columns on the periodic table are called groups or families. 3. The Periodic Table is arranged by increasing atomic number. __. 4. Describe the location of metals, nonmetals, and metalloids on the periodic table. Metal ...
Document
... Lanthanides and actinides “rare earth elements” subset of transition metals… Common metals: Fe, Cu, Ni, Zn… In nature: typically found as compounds • In elemental form in nature: Ag, Au, Pt (…quite unreactive) Most react with air (oxidation), but not violently: rusting of Fe • Most elemental t ...
... Lanthanides and actinides “rare earth elements” subset of transition metals… Common metals: Fe, Cu, Ni, Zn… In nature: typically found as compounds • In elemental form in nature: Ag, Au, Pt (…quite unreactive) Most react with air (oxidation), but not violently: rusting of Fe • Most elemental t ...
The Periodic Table
... Valence Electrons • Valence electrons are the electrons found in the outermost energy level. • Example: Carbon has 4 valence electrons ...
... Valence Electrons • Valence electrons are the electrons found in the outermost energy level. • Example: Carbon has 4 valence electrons ...
PeriodicTableNotes
... Every periodic table will have a square for each element with the atomic number, atomic mass, element name, and the element symbol. o The _______________ _______________ is the symbol given to represent that particular element of that square. o The ______________ ________________ is the actual name ...
... Every periodic table will have a square for each element with the atomic number, atomic mass, element name, and the element symbol. o The _______________ _______________ is the symbol given to represent that particular element of that square. o The ______________ ________________ is the actual name ...
atomic number
... 3- The strongest non-metallic element lies in group ----------------. ( 7A) 4- ------------------- lie preceding noble gases in the periodic table, and during the chemical reaction they form -------------------ions. ( non metals - -ve ions ) ...
... 3- The strongest non-metallic element lies in group ----------------. ( 7A) 4- ------------------- lie preceding noble gases in the periodic table, and during the chemical reaction they form -------------------ions. ( non metals - -ve ions ) ...
here
... periodic table identify elements by group number identify metals, non-metals, transition metals, lanthanides, actinides, alkali metals, alkaline earths, halogens, noble gases know the names and symbols for elements 1 – 20, Cr, Fe, Ni, Cu, Zn, Ag, Sn, W, Pt, Au, Hg, Pb atomic mass calculate average a ...
... periodic table identify elements by group number identify metals, non-metals, transition metals, lanthanides, actinides, alkali metals, alkaline earths, halogens, noble gases know the names and symbols for elements 1 – 20, Cr, Fe, Ni, Cu, Zn, Ag, Sn, W, Pt, Au, Hg, Pb atomic mass calculate average a ...
Periodic Table and Elements Review
... 18.) In one of labs we looked investigated the reactivity of aluminum, magnesium, and calcium by immersing them in water. What was the order from most to least reactive? ...
... 18.) In one of labs we looked investigated the reactivity of aluminum, magnesium, and calcium by immersing them in water. What was the order from most to least reactive? ...
Study Guide – Honors Chemistry: Exam One
... Based on ionic charge, be able to predict the ratio with which anions and cations will combine to form a compound. Predict the formula for the following ionic compounds (use your book to remind yourself of some of the polyatomic ions – you will be given a list of names and formulas for polyatomic io ...
... Based on ionic charge, be able to predict the ratio with which anions and cations will combine to form a compound. Predict the formula for the following ionic compounds (use your book to remind yourself of some of the polyatomic ions – you will be given a list of names and formulas for polyatomic io ...
The Periodic Table
... familiar: copper, tin, zinc, iron, nickel, gold, and silver. They are good conductors of heat and electricity. ...
... familiar: copper, tin, zinc, iron, nickel, gold, and silver. They are good conductors of heat and electricity. ...
File
... 2. Identify each element as a metal, metalloid, or nonmetal. a) fluorine b) germanium c) zinc d) phosphorous e) lithium 3. Give two examples of elements for each category. a) noble gases b) halogens c) alkali metals d) alkaline earth metals 4. The halogen family or halides form salts with which othe ...
... 2. Identify each element as a metal, metalloid, or nonmetal. a) fluorine b) germanium c) zinc d) phosphorous e) lithium 3. Give two examples of elements for each category. a) noble gases b) halogens c) alkali metals d) alkaline earth metals 4. The halogen family or halides form salts with which othe ...
File - chemistryattweed
... Note: Group 8 the noble gases is also known as Group 0 as these elements have a valency of zero. Electronegativity The electronegativity of an element is a measure of the ability of the atom of that element to attract bonding electrons towards itself when it forms compounds. Electronegativity increa ...
... Note: Group 8 the noble gases is also known as Group 0 as these elements have a valency of zero. Electronegativity The electronegativity of an element is a measure of the ability of the atom of that element to attract bonding electrons towards itself when it forms compounds. Electronegativity increa ...
PART 1: ATOMIC NUMBER - hrsbstaff.ednet.ns.ca
... 2. Find all of the gasses in the periodic table and with a black pencil crayon, outline each of them on the above table. 3. In the table below, indicate which gasses are located in each of the periods. Your table will not have elements in every blank space. ...
... 2. Find all of the gasses in the periodic table and with a black pencil crayon, outline each of them on the above table. 3. In the table below, indicate which gasses are located in each of the periods. Your table will not have elements in every blank space. ...
Test 1. 2nd prep. ques
... 2- The measuring unit of atomic radius which is used to measure the atomic size.(--picometer-) 3- Elements present in group zero.( noble gases ) 4- It is the series in which metals are arranged in a descending order according to their chemical activity . (--chemical activity series ) 5- Oxides of me ...
... 2- The measuring unit of atomic radius which is used to measure the atomic size.(--picometer-) 3- Elements present in group zero.( noble gases ) 4- It is the series in which metals are arranged in a descending order according to their chemical activity . (--chemical activity series ) 5- Oxides of me ...
Compare in detail Democritus and Aristotle`s theories on matter
... What are the 3 subatomic What is the atomic number and why is particles that make up an it important? atom? What are their charges? ...
... What are the 3 subatomic What is the atomic number and why is particles that make up an it important? atom? What are their charges? ...
The periodic table
... atomic mass shown on the periodic table is actually an average of the various isotopes that exist. When making calculations, we round to the nearest whole number. ...
... atomic mass shown on the periodic table is actually an average of the various isotopes that exist. When making calculations, we round to the nearest whole number. ...