More Chemistry!
... useful way The first Periodic Table of Elements was proposed by a Russian – Dmitri Mendeleev in ...
... useful way The first Periodic Table of Elements was proposed by a Russian – Dmitri Mendeleev in ...
Name - TeacherWeb
... 10. Circle the letter of each sentence that is true about a carbon-12 atom. a. It has 6 protons and 6 neutrons. b. Scientists assigned a mass of 6 atomic mass units to the carbon-12 atom. c. It is used as a standard for comparing the masses of atoms. d. An atomic mass unit is defined as one twelfth ...
... 10. Circle the letter of each sentence that is true about a carbon-12 atom. a. It has 6 protons and 6 neutrons. b. Scientists assigned a mass of 6 atomic mass units to the carbon-12 atom. c. It is used as a standard for comparing the masses of atoms. d. An atomic mass unit is defined as one twelfth ...
the periodic table
... • elements are arranged in increasing order of atomic number, as you go from left to right across the table. – The vertical columns are called "groups“ or “families”. – The horizontal rows are called "periods". ...
... • elements are arranged in increasing order of atomic number, as you go from left to right across the table. – The vertical columns are called "groups“ or “families”. – The horizontal rows are called "periods". ...
File
... [Nitrogen, Oxygen, Argon] has a full valence shell and is a noble gas. Noble gases are [inert, very reactive, only react with certain elements]. [Potassium, Calcium, Sulfur, Neon] has properties most similar to oxygen. [Calcium, Potassium, Chlorine, Sodium] has two valence electrons. Periods form [h ...
... [Nitrogen, Oxygen, Argon] has a full valence shell and is a noble gas. Noble gases are [inert, very reactive, only react with certain elements]. [Potassium, Calcium, Sulfur, Neon] has properties most similar to oxygen. [Calcium, Potassium, Chlorine, Sodium] has two valence electrons. Periods form [h ...
Atoms, elements and compounds
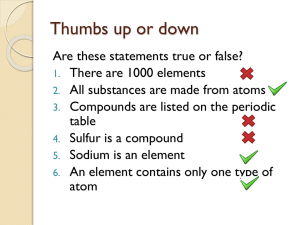
... Are these statements true or false? 1. There are 1000 elements 2. All substances are made from atoms 3. Compounds are listed on the periodic table 4. Sulfur is a compound 5. Sodium is an element 6. An element contains only one type of atom ...
... Are these statements true or false? 1. There are 1000 elements 2. All substances are made from atoms 3. Compounds are listed on the periodic table 4. Sulfur is a compound 5. Sodium is an element 6. An element contains only one type of atom ...
Periodic Table Intro - Chemistry Hunger Games
... Element Name and Symbol • Every element has a 1 or 2 letter symbol. • The first letter is ALWAYS capitalized. ...
... Element Name and Symbol • Every element has a 1 or 2 letter symbol. • The first letter is ALWAYS capitalized. ...
Chapter6
... Pre-class Questions 1/7 How many valence electrons does Antimony (Sb) have? What is it’s Lewis Dot structure? ...
... Pre-class Questions 1/7 How many valence electrons does Antimony (Sb) have? What is it’s Lewis Dot structure? ...
Preview Sample 1
... Understand the experiments used to determine the structure of the atom. Compare the properties of protons, electrons, and neutrons. Determine the number of protons, electrons, and neutrons for any atom. Explain the difference between isotopes of a given element. Determine the atomic number ...
... Understand the experiments used to determine the structure of the atom. Compare the properties of protons, electrons, and neutrons. Determine the number of protons, electrons, and neutrons for any atom. Explain the difference between isotopes of a given element. Determine the atomic number ...
The Periodic Table of Elements - PAMS-Doyle
... • They have been moved to the bottom to make the periodic table easier to read • First row is the lanthanide series, shiny, soft, malleable metals, that are conductive • The second row is the actinide series, all are radioactive and only the first four are present in nature • Elements numbered 92-11 ...
... • They have been moved to the bottom to make the periodic table easier to read • First row is the lanthanide series, shiny, soft, malleable metals, that are conductive • The second row is the actinide series, all are radioactive and only the first four are present in nature • Elements numbered 92-11 ...
Chemistry 101 Topic 4
... have specific arrangements because they are held together by a:rac2ve forces called bonds. A bond is the result of coulombic a:rac2on between posi2vely charged nuclei and nega2vely charged electrons. We will ...
... have specific arrangements because they are held together by a:rac2ve forces called bonds. A bond is the result of coulombic a:rac2on between posi2vely charged nuclei and nega2vely charged electrons. We will ...
Periodic Table Worksheet
... 8. As you go down a group, the first ionization energy generally (DECREASES / increases). Why? OUTERMOST ELECTRON IS FARTHER AWAY FROM NUCLEUS; SHIELDING EFFECT OF INNER ELECTRONS. 9. Where is the highest electronegativity found? UPPER RIGHT (F) 10. Where is the lowest electronegativity found? LOWER ...
... 8. As you go down a group, the first ionization energy generally (DECREASES / increases). Why? OUTERMOST ELECTRON IS FARTHER AWAY FROM NUCLEUS; SHIELDING EFFECT OF INNER ELECTRONS. 9. Where is the highest electronegativity found? UPPER RIGHT (F) 10. Where is the lowest electronegativity found? LOWER ...
Periodic Table Funsheet (KEY) 1. Where are the most active metals
... 8. As you go down a group, the first ionization energy generally (DECREASES / increases). Why? OUTERMOST ELECTRON IS FARTHER AWAY FROM NUCLEUS; SHIELDING EFFECT OF INNER ELECTRONS. 9. Where is the highest electronegativity found? UPPER RIGHT (F) 10. Where is the lowest electronegativity found? LOWER ...
... 8. As you go down a group, the first ionization energy generally (DECREASES / increases). Why? OUTERMOST ELECTRON IS FARTHER AWAY FROM NUCLEUS; SHIELDING EFFECT OF INNER ELECTRONS. 9. Where is the highest electronegativity found? UPPER RIGHT (F) 10. Where is the lowest electronegativity found? LOWER ...
ORGANIZATION OF THE PERIODIC TABLE
... Groups 13-16 = BCNO group 3-6 valence electrons Group 17 = Halogens (combine to form salts) 7 valence electrons Group 18 = Nobel Gases (least reactive) 8 valence electrons Lanthanides & Actinides - Many are radioactive, also called rare earth metals ...
... Groups 13-16 = BCNO group 3-6 valence electrons Group 17 = Halogens (combine to form salts) 7 valence electrons Group 18 = Nobel Gases (least reactive) 8 valence electrons Lanthanides & Actinides - Many are radioactive, also called rare earth metals ...
AP Chemistry-Chapter 6 MC Questions
... The ion with the highest atomic number bears the highest positive charge. The ion with the lowest atomic number bears the least negative charge. The ion with a 1- charge is obtained by adding one electron to a Group VIIA element. All the ions have a noble gas electronic configuration. II and III I, ...
... The ion with the highest atomic number bears the highest positive charge. The ion with the lowest atomic number bears the least negative charge. The ion with a 1- charge is obtained by adding one electron to a Group VIIA element. All the ions have a noble gas electronic configuration. II and III I, ...
Key Words Isotope- Atoms with the same number of protons but
... Isotope- Atoms with the same number of protons but different numbers of neutrons. Atomic Mass- The average mass of all the isotopes of that element. Mass Number- Sum of protons and neutrons. Atomic Number- The number of protons in an atom. ...
... Isotope- Atoms with the same number of protons but different numbers of neutrons. Atomic Mass- The average mass of all the isotopes of that element. Mass Number- Sum of protons and neutrons. Atomic Number- The number of protons in an atom. ...
Chapter 4- The Periodic Table Section 1
... table and Henry Moseley found a different physical basis for the arrangement of elements ...
... table and Henry Moseley found a different physical basis for the arrangement of elements ...
Powerpoint - Valence Electrons
... • Compound – 2 or more elements joined (e.g. H2O). • Molecule – 2 or more atoms combined (e.g. CO carbon monoxide; O2 oxygen gas). ...
... • Compound – 2 or more elements joined (e.g. H2O). • Molecule – 2 or more atoms combined (e.g. CO carbon monoxide; O2 oxygen gas). ...
Matter: Building Blocks of the Universe Chapter 5 Classification of
... o Elements within the same family have similar but not identical properties Each horizontal row of elements is called a period o Elements in a period are not alike in properties Each element is given a separate square—contains atomic number, atomic mass, chemical symbol, and name Dark zipzag l ...
... o Elements within the same family have similar but not identical properties Each horizontal row of elements is called a period o Elements in a period are not alike in properties Each element is given a separate square—contains atomic number, atomic mass, chemical symbol, and name Dark zipzag l ...
NAME: Unit 3 Test Review Arsenic (As), Selenium (Se), and
... 8. What are the three main subatomic particles of an atom? protons, neutrons, electrons 9. Which group on the periodic table is made of ONLY gases? group 18, noble gases 10. Which two groups on the periodic table are HIGHLY REACTIVE? group 1(alkali metals) and group 17 (halogens) 11. Which group is ...
... 8. What are the three main subatomic particles of an atom? protons, neutrons, electrons 9. Which group on the periodic table is made of ONLY gases? group 18, noble gases 10. Which two groups on the periodic table are HIGHLY REACTIVE? group 1(alkali metals) and group 17 (halogens) 11. Which group is ...
Study Guide for Quiz on Tuesday February 26th - seys
... Periodic table- a table of the elements arranged by atomic number that shows a pattern in their properties Group- a vertical column in the periodic table of the elements, elements have similar properties Period- a horizontal row in the periodic table of the elements, elements have varying propertie ...
... Periodic table- a table of the elements arranged by atomic number that shows a pattern in their properties Group- a vertical column in the periodic table of the elements, elements have similar properties Period- a horizontal row in the periodic table of the elements, elements have varying propertie ...
Chapter 5
... • Left blank spots in table which predicted properties of elements not yet discovered ...
... • Left blank spots in table which predicted properties of elements not yet discovered ...