Chapter 6 Study Guide
... a. period 3, Group IIIA _____________________________________________ b. period 1, Group VIIIA ____________________________________________ c. period 4, Group IIA ______________________________________________ d. period 6, Group VA ______________________________________________ 7. How many Valence e ...
... a. period 3, Group IIIA _____________________________________________ b. period 1, Group VIIIA ____________________________________________ c. period 4, Group IIA ______________________________________________ d. period 6, Group VA ______________________________________________ 7. How many Valence e ...
assignment-07-a3
... (Metallic character increases toward the bottom left side of the periodic table.) 15- Which of the following is not a property of an alkali metal? a) Alkali metals are highly reactive towards oxygen and water. b) Alkali metals can be cut with a dull knife. c) Alkali metals have relatively low meltin ...
... (Metallic character increases toward the bottom left side of the periodic table.) 15- Which of the following is not a property of an alkali metal? a) Alkali metals are highly reactive towards oxygen and water. b) Alkali metals can be cut with a dull knife. c) Alkali metals have relatively low meltin ...
Periodic Table
... 1. In the past people believed that there were only 4 elements now we know that there are over 100 elements 2. Everything is made of elements 3. A reason of your own. ...
... 1. In the past people believed that there were only 4 elements now we know that there are over 100 elements 2. Everything is made of elements 3. A reason of your own. ...
4.1 Vocabulary
... An atom of iron contains 26 protons, so the atomic number of iron is 26. Atomic number is used in identifying atoms. element a pure substance made of only one type of atom Copper, helium, calcium, and neon are all types of elements. Each element is made up of one kind of atom. A copper atom is diffe ...
... An atom of iron contains 26 protons, so the atomic number of iron is 26. Atomic number is used in identifying atoms. element a pure substance made of only one type of atom Copper, helium, calcium, and neon are all types of elements. Each element is made up of one kind of atom. A copper atom is diffe ...
Objective - davis.k12.ut.us
... a. Dmitri Mendeleev created the first periodic table in 1869. He discovered that the elements shared certain patterns and trends. He observed that the elements shared some chemical and physical properties. Dmitri believed the properties that the elements shared were important. Therefore, he wrote do ...
... a. Dmitri Mendeleev created the first periodic table in 1869. He discovered that the elements shared certain patterns and trends. He observed that the elements shared some chemical and physical properties. Dmitri believed the properties that the elements shared were important. Therefore, he wrote do ...
The Periodic Table Notes
... Objective 1. Students will name who first created the periodic table and when it was created. 2. Students will describe the properties Mendeleev used to create the periodic table. 3. Students will define atomic mass, atomic mass unit, and atomic number. 4. Students will define valence electrons, pro ...
... Objective 1. Students will name who first created the periodic table and when it was created. 2. Students will describe the properties Mendeleev used to create the periodic table. 3. Students will define atomic mass, atomic mass unit, and atomic number. 4. Students will define valence electrons, pro ...
5.2 The Modern Periodic Table
... electrons) – similar electron configurations – similar chemical properties ...
... electrons) – similar electron configurations – similar chemical properties ...
Periodic Trends
... Periodic Law – there is a periodic repetition of physical and chemical properties when elements are arranged by increasing atomic Some of Medeleev’s work (1869) number ...
... Periodic Law – there is a periodic repetition of physical and chemical properties when elements are arranged by increasing atomic Some of Medeleev’s work (1869) number ...
Organizing Clutter: The Organizing of the Elements of the Universe
... periodic table. You will work with a partner and each of you will build at least one atom of an element. Each of you will have: At least one element card with information about the element Paper plates – one for each element 3 colors of label dots - ________ represents Protons; __________ represents ...
... periodic table. You will work with a partner and each of you will build at least one atom of an element. Each of you will have: At least one element card with information about the element Paper plates – one for each element 3 colors of label dots - ________ represents Protons; __________ represents ...
Chapter 6 Studyguide: The Periodic Table
... 25.What factors contribute to the increase in atomic size within a group in the periodic table as the atomic ...
... 25.What factors contribute to the increase in atomic size within a group in the periodic table as the atomic ...
5SC19 Elements, Mixtures, and Compounds
... All atoms have a nucleus. The nucleus usually has neutrons and protons. Neutrons have no electrical charge and protons have a positive charge. An atom is identified by its number of protons, and that number is unique to that atom. For example, sodium has 11 protons, which means NO other atom has 11 ...
... All atoms have a nucleus. The nucleus usually has neutrons and protons. Neutrons have no electrical charge and protons have a positive charge. An atom is identified by its number of protons, and that number is unique to that atom. For example, sodium has 11 protons, which means NO other atom has 11 ...
Atomic
... - The symbol for the positive ion (metal) is written first - The symbol for the negative ion (non-metal) is written second. - The charges on each ion are criss-crossed and used as subscripts for the formula but the positive and negative signs are never used in the subscripts. - If the subscripts can ...
... - The symbol for the positive ion (metal) is written first - The symbol for the negative ion (non-metal) is written second. - The charges on each ion are criss-crossed and used as subscripts for the formula but the positive and negative signs are never used in the subscripts. - If the subscripts can ...
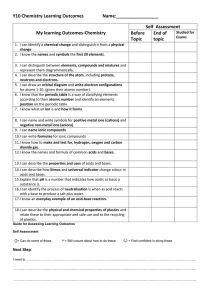
Y10 Chem SLOs 2016 File
... 4. I can describe the structure of the atom, including protons, neutrons and electrons. 5. I can draw an orbital diagram and write electron configurations for atoms 1-20. (given their atomic number). 6. I know that the periodic table is a way of classifying elements according to their atomic number ...
... 4. I can describe the structure of the atom, including protons, neutrons and electrons. 5. I can draw an orbital diagram and write electron configurations for atoms 1-20. (given their atomic number). 6. I know that the periodic table is a way of classifying elements according to their atomic number ...
pg156
... a. How do the first ionization energies of main-group elements vary across a period and down a group? ...
... a. How do the first ionization energies of main-group elements vary across a period and down a group? ...
Unit 1 Matter: Properties and Change
... second column (group two) has two electrons in the outer shell. Transition elements (ones in the middle) are different. ...
... second column (group two) has two electrons in the outer shell. Transition elements (ones in the middle) are different. ...
The Modern Periodic Table
... Nonmetals are elements that are poor conductors of heat and electric current. 3. Metalloids Metalloids are elements with properties that fall between those metals and nonmetals. d. Variation across a Period Across a period from left to right, the elements become less metallic and more nonmetallic in ...
... Nonmetals are elements that are poor conductors of heat and electric current. 3. Metalloids Metalloids are elements with properties that fall between those metals and nonmetals. d. Variation across a Period Across a period from left to right, the elements become less metallic and more nonmetallic in ...
Ch 6.1 and 6.2 Review
... Compare and contrast the electron configurations of elements within the same family and elements within the same period. Look particularly at sublevels and energy levels. ...
... Compare and contrast the electron configurations of elements within the same family and elements within the same period. Look particularly at sublevels and energy levels. ...
Isotope - MrKanesSciencePage
... 4. First stated that atoms of different elements have different masses. 5. His model of the atom is often called the “planetary” model 6. Discovered that most of the mass and positive charge of an atom is located in the center. 7. Shot positive alpha particles at a very thin sheet of gold foil. 8. E ...
... 4. First stated that atoms of different elements have different masses. 5. His model of the atom is often called the “planetary” model 6. Discovered that most of the mass and positive charge of an atom is located in the center. 7. Shot positive alpha particles at a very thin sheet of gold foil. 8. E ...
Midterm Review 2013
... Which orbital corresponds to the transition metals in the periodic table? ...
... Which orbital corresponds to the transition metals in the periodic table? ...
Key Terms: 1. Molecule- An atom 2. Brainstorm
... 2. Brainstorm- using your own brain and thinking outside of the box 3. Volume (in chemistry)- the amount in side 4. Periodic table of elements- Helps know the atomic mass and protons 5. Element (in chemistry)- A SUBSTANCE 6. Atomic mass- how much a substance mass is. Found on the periodic table 7. M ...
... 2. Brainstorm- using your own brain and thinking outside of the box 3. Volume (in chemistry)- the amount in side 4. Periodic table of elements- Helps know the atomic mass and protons 5. Element (in chemistry)- A SUBSTANCE 6. Atomic mass- how much a substance mass is. Found on the periodic table 7. M ...