* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Isotope - MrKanesSciencePage
Survey
Document related concepts
Transcript
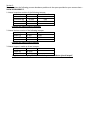
Name: ___________________________________ Date: ___________ Pd: _____ KANE Atomic Theory, Atomic Dimensions, Isotopes, and Periodic Table Quiz Section 1: Match the following people with their accomplishments: A. Dalton B. Thomson C. Rutherford 1. Discovered the electron 2. Discovered the nucleus 3. His model of the atom is often referred to as the “plum pudding” model. 4. First stated that atoms of different elements have different masses. 5. His model of the atom is often called the “planetary” model 6. Discovered that most of the mass and positive charge of an atom is located in the center. 7. Shot positive alpha particles at a very thin sheet of gold foil. 8. Experimented with cathode ray tubes to observe how beams of electrons were affected by magnetic fields. 9. Stated that atoms of the same element had the same mass. 10. Stated that during a chemical reaction, atoms just rearranged – no new atoms or elements were created. Section 2: Please fill in the following table with the appropriate responses. Isotope notation (A) Atomic number Protons Neutrons Mass number Electrons Charge 12 (E) (B) (F) 13 (G) (C) (H) (D) (I) +2 (J) (K) (O) (L) 26 92 (P) 146 (Q) (M) 56 90 (R) (N) 0 Section 3: Please complete the following percent abundance problems in the space provided on your answer sheet. – SHOW YOUR WORK!!!!! 1. Natural strontium consists of the following isotopes: Isotope Mass Percent Abundance Strontium-84 83.913 0.56 Strontium-86 85.909 9.86 Strontium-87 86.909 7.00 Strontium-88 87.906 82.58 Calculate the atomic mass of strontium. 2. Natural kanium consists of the following isotopes: Isotope Mass Percent Abundance Kanium-74 73.910 72.36 Kanium-77 76.908 16.92 Kanium-78 77.907 10.72 Calculate the atomic mass of kanium 3. Natural copper is made up of two isotopes: Isotope Mass Percent Abundance Copper-63 62.9296 Copper-65 64.9278 Copper's atomic weight of 63.546, what is the percent abundance of each isotope? Section 4: Parts of the periodic table Answer the following questions based on the periodic table below. 1. Fill the box with the pattern for a transition metal 2. Fill the box with the pattern for a nonmetal 3. Fill the box with the pattern for a metalloid (semimetal) 4. Fill the box with the pattern for an actinide/lanthanide series element. 5. Which direction do the families/groups run (up and down, or left to right?) 6. Which direction do the periods run (up and down, or left to right?) 7. What is the special name given to the elements in the first column? 8. What is the special name given to the elements in the second column? 9. What is the name given to the elements in the 17 th column? 10. What is the name given to the elements in the 18th column? Bonus: Please use the space provided on your answer sheet, as well as the conversions found here, to answer the following question. 1 day = 24hrs 1hr = 60 min 1 min = 60 sec 1L = 0.246 gal My indoor Olympic swimming pool (2500KL) developed a leak while I was vacationing on my yacht off of the coast of Marseille, France this summer. Upon my return home, I discovered, to my dismay, that my pool was completely drained. If my hose delivers water (Evian –of course!) at a rate of 5 gallons/min, how many days will it take to refill my pool?