Lewis Reeve Gibbes and the Classification of the Elements
... easier for Gibbes to arrive at a logical arrangement. The best that he could do was to lump all but hydrogen and mercury in a second part of his table, beneath the positive portion of the main part but distinct from it. Here he grouped the twenty recalcitrant metals' along lines suggested by analyti ...
... easier for Gibbes to arrive at a logical arrangement. The best that he could do was to lump all but hydrogen and mercury in a second part of his table, beneath the positive portion of the main part but distinct from it. Here he grouped the twenty recalcitrant metals' along lines suggested by analyti ...
Chapter 5 PRACTICE TEST
... c. electrons exhibit properties of both particles and waves. d. the chemical properties of elements can be grouped according to periodicity but physical properties cannot. Elements in a group or column in the periodic table can be expected to have similar a. atomic masses. c. numbers of neutrons. b. ...
... c. electrons exhibit properties of both particles and waves. d. the chemical properties of elements can be grouped according to periodicity but physical properties cannot. Elements in a group or column in the periodic table can be expected to have similar a. atomic masses. c. numbers of neutrons. b. ...
Class XI worksheet - Indian School Muscat
... 11. What are isoelectronic species? Name the species that will be isoelectronic with each of the following atoms or ions. (i) F- (ii) Ar ...
... 11. What are isoelectronic species? Name the species that will be isoelectronic with each of the following atoms or ions. (i) F- (ii) Ar ...
Chapter 6: The Periodic Table and Periodic Law
... arrangement of the elements with the modern periodic table on the inside back cover of your textbook, you’ll see that some of his rows correspond to columns on the modern periodic table. Acceptance of the law of octaves was hampered because the law did not work for all of the known elements. Also, u ...
... arrangement of the elements with the modern periodic table on the inside back cover of your textbook, you’ll see that some of his rows correspond to columns on the modern periodic table. Acceptance of the law of octaves was hampered because the law did not work for all of the known elements. Also, u ...
Classification and Periodic Properties of Elements
... could be predicted with a fair accuracy. For example, both gallium and germanium were not discovered at the time when Mendeleev proposed his periodic table. Mendeleevnamed these elements as Eka-Aluminium and Eka·Silicon respectively. Later on, when these elements were discovered, Mendeleev's predict ...
... could be predicted with a fair accuracy. For example, both gallium and germanium were not discovered at the time when Mendeleev proposed his periodic table. Mendeleevnamed these elements as Eka-Aluminium and Eka·Silicon respectively. Later on, when these elements were discovered, Mendeleev's predict ...
Lorna Merklinger
... SC.8.P.8.5#: Recognize that there are a finite number of elements and that their atoms combine in a multitude of ways to produce compounds that make up all of the living and nonliving things that we Benchmark ...
... SC.8.P.8.5#: Recognize that there are a finite number of elements and that their atoms combine in a multitude of ways to produce compounds that make up all of the living and nonliving things that we Benchmark ...
The Modern Periodic Table
... • The periodic law states that when the elements are arranged by increasing atomic number, there is a periodic repetition of their chemical and physical properties. • The periodic table organizes the elements into periods (rows) and groups (columns); elements with similar properties are in the same ...
... • The periodic law states that when the elements are arranged by increasing atomic number, there is a periodic repetition of their chemical and physical properties. • The periodic table organizes the elements into periods (rows) and groups (columns); elements with similar properties are in the same ...
Chapter 6: The Periodic Table
... • The periodic law states that when the elements are arranged by increasing atomic number, there is a periodic repetition of their chemical and physical properties. • The periodic table organizes the elements into periods (rows) and groups (columns); elements with similar properties are in the same ...
... • The periodic law states that when the elements are arranged by increasing atomic number, there is a periodic repetition of their chemical and physical properties. • The periodic table organizes the elements into periods (rows) and groups (columns); elements with similar properties are in the same ...
Chemistry: Matter and Change
... • The periodic law states that when the elements are arranged by increasing atomic number, there is a periodic repetition of their chemical and physical properties. • The periodic table organizes the elements into periods (rows) and groups (columns); elements with similar properties are in the same ...
... • The periodic law states that when the elements are arranged by increasing atomic number, there is a periodic repetition of their chemical and physical properties. • The periodic table organizes the elements into periods (rows) and groups (columns); elements with similar properties are in the same ...
Science - ExamResults.net
... In addition to this, solved and practice problems are included which not only aim at covering the topic but also make students ready to face the competition. The topic-wise classified “question and answer” format of this book helps students in easy comprehension. Numerical problems included at the e ...
... In addition to this, solved and practice problems are included which not only aim at covering the topic but also make students ready to face the competition. The topic-wise classified “question and answer” format of this book helps students in easy comprehension. Numerical problems included at the e ...
Std 10th, Science and Technology, Maharashtra Board, English
... In addition to this, solved and practice problems are included which not only aim at covering the topic but also make students ready to face the competition. The topic-wise classified “question and answer” format of this book helps students in easy comprehension. Numerical problems included at the e ...
... In addition to this, solved and practice problems are included which not only aim at covering the topic but also make students ready to face the competition. The topic-wise classified “question and answer” format of this book helps students in easy comprehension. Numerical problems included at the e ...
The Periodic Table
... The Periodic Table: Periods The periodic table provides information about the locations of electrons in an atomic of an element based on the period in which the element appears. There are seven periods in the periodic table. The two rows at the bottom of the table are actually parts of Period ...
... The Periodic Table: Periods The periodic table provides information about the locations of electrons in an atomic of an element based on the period in which the element appears. There are seven periods in the periodic table. The two rows at the bottom of the table are actually parts of Period ...
File
... Mendeleev placed the elements in order of increasing atomic mass and then noticed a repeating pattern in the oxide and hydride formula. ...
... Mendeleev placed the elements in order of increasing atomic mass and then noticed a repeating pattern in the oxide and hydride formula. ...
The Periodic Law - Mona Shores Blogs
... mass provided chemists with a convenient way to organize the elements. At the same time, it was recognized that there were certain elements that had similar chemical properties. Mendeleev arranged the elements in rows according to atomic weight and kept elements with similar chemical properties in t ...
... mass provided chemists with a convenient way to organize the elements. At the same time, it was recognized that there were certain elements that had similar chemical properties. Mendeleev arranged the elements in rows according to atomic weight and kept elements with similar chemical properties in t ...
The Periodic Table
... By the year 1700, only a handful of elements had been identified and isolated. Several of these, such as copper and lead, had been known since ancient times. As scientific methods improved, the rate of discovery dramatically increased (Figure 1.1). With the ever-increasing number of elements, chemis ...
... By the year 1700, only a handful of elements had been identified and isolated. Several of these, such as copper and lead, had been known since ancient times. As scientific methods improved, the rate of discovery dramatically increased (Figure 1.1). With the ever-increasing number of elements, chemis ...
C H A P T E R
... compounds are white solids that dissolve in water to form solutions that conduct electricity. Similarly, the elements fluorine, chlorine, bromine, and iodine can combine with sodium in a 1:1 ratio to form NaF, NaCl, NaBr, and NaI. These compounds are also white solids that can dissolve in water to f ...
... compounds are white solids that dissolve in water to form solutions that conduct electricity. Similarly, the elements fluorine, chlorine, bromine, and iodine can combine with sodium in a 1:1 ratio to form NaF, NaCl, NaBr, and NaI. These compounds are also white solids that can dissolve in water to f ...
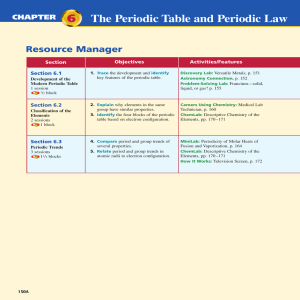
Chapter 6: The Periodic Table and Periodic Law
... who is shown in Figure 6-2, proposed an organization scheme for the elements. Newlands noticed that when the elements were arranged by increasing atomic mass, their properties repeated every eighth element. In other words, the first and eighth elements had similar properties, the second and ninth el ...
... who is shown in Figure 6-2, proposed an organization scheme for the elements. Newlands noticed that when the elements were arranged by increasing atomic mass, their properties repeated every eighth element. In other words, the first and eighth elements had similar properties, the second and ninth el ...
Chapter 6: The Periodic Table and Periodic Law
... oranges, and peaches were mixed into one bin at the grocery store. Organizing things according to their properties is often useful. Scientists organize the many different types of chemical elements in the periodic table. ...
... oranges, and peaches were mixed into one bin at the grocery store. Organizing things according to their properties is often useful. Scientists organize the many different types of chemical elements in the periodic table. ...
ch 3 classification of elements and periodic properties
... 1. in group 2 → filled ns subshells 2. in group 15 → half-filled np subshells 3. in group 18 → fully filled subshells These elec config are relatively stable & hence these have +ve or very low –ve EGEs. ...
... 1. in group 2 → filled ns subshells 2. in group 15 → half-filled np subshells 3. in group 18 → fully filled subshells These elec config are relatively stable & hence these have +ve or very low –ve EGEs. ...
The Periodic Table and Periodic Law
... peaches were mixed into one bin at the grocery store. Organizing things according to their properties is often useful. Scientists organize the many different types of chemical elements in the periodic table. ...
... peaches were mixed into one bin at the grocery store. Organizing things according to their properties is often useful. Scientists organize the many different types of chemical elements in the periodic table. ...
The Periodic Table and The Periodic Law
... negatively charged depending if the number of electrons in an atom is greater or less then the number of protons in the atom. ...
... negatively charged depending if the number of electrons in an atom is greater or less then the number of protons in the atom. ...
Periodic Table ppt
... • The periodic law states that when the elements are arranged by increasing atomic number, there is a periodic repetition of their chemical and physical properties. • The periodic table organizes the elements into periods (rows) and groups (columns); elements with similar properties are in the same ...
... • The periodic law states that when the elements are arranged by increasing atomic number, there is a periodic repetition of their chemical and physical properties. • The periodic table organizes the elements into periods (rows) and groups (columns); elements with similar properties are in the same ...
Minimum electrophilicity principle in Lewis acid–base complexes of
... 2. Results and discussion To reinvestigate the acidity strength of some boron trihalides (BX3; X = F, Cl and Br) from theoretical point of view, two sets of Lewis bases (weak and strong), which can form stable compounds with these acids, are considered here. It is expected that more stable complexes ...
... 2. Results and discussion To reinvestigate the acidity strength of some boron trihalides (BX3; X = F, Cl and Br) from theoretical point of view, two sets of Lewis bases (weak and strong), which can form stable compounds with these acids, are considered here. It is expected that more stable complexes ...
Atomic Structure and the Periodic Table
... 6. The staircase line divides the metals, on the left, from the nonmentals, on the right. 7. a. The metalloids are located along the bold staircase. b. Examples of metalloids include aluminum and boron. c. A metalloid is an elements that has properties common to both metals and nonmetals. 8. As you ...
... 6. The staircase line divides the metals, on the left, from the nonmentals, on the right. 7. a. The metalloids are located along the bold staircase. b. Examples of metalloids include aluminum and boron. c. A metalloid is an elements that has properties common to both metals and nonmetals. 8. As you ...
Usefulness of the periodic table in studying the chemistry of elements:
... This family consists of the elements: oxygen (O), sulfur (S), selenium (Se), tellurium (Te) and polonium (Po). The Group 16 elements are also known as “chalcogens”. Polonium is the only true metal; Te is a semi-metal; oxygen, sulfur and selenium are non-metals. The general valence electron configura ...
... This family consists of the elements: oxygen (O), sulfur (S), selenium (Se), tellurium (Te) and polonium (Po). The Group 16 elements are also known as “chalcogens”. Polonium is the only true metal; Te is a semi-metal; oxygen, sulfur and selenium are non-metals. The general valence electron configura ...