FREE Sample Here
... Global Outcomes: G7 Demonstrate the ability to make connections between concepts across chemistry. 29) According to the atomic theory, ________. A) all atoms are different B) atoms are neither created nor destroyed during a chemical reaction C) atoms of the same element combine to form compounds D) ...
... Global Outcomes: G7 Demonstrate the ability to make connections between concepts across chemistry. 29) According to the atomic theory, ________. A) all atoms are different B) atoms are neither created nor destroyed during a chemical reaction C) atoms of the same element combine to form compounds D) ...
Review 4-9 - Lakeland Regional High School
... ____ 43. As you move from left to right across the second period of the periodic table ____. a. ionization energy increases c. electronegativity decreases b. atomic radii increase d. atomic mass decreases ____ 44. Of the following elements, which one has the smallest first ionization energy? a. boro ...
... ____ 43. As you move from left to right across the second period of the periodic table ____. a. ionization energy increases c. electronegativity decreases b. atomic radii increase d. atomic mass decreases ____ 44. Of the following elements, which one has the smallest first ionization energy? a. boro ...
Periodic trends
... Ionization energy vs. atomic number graph Chapter 55 Section Section 11 History History of of the the Chapter Periodic Table Table pages pages 133-137 ...
... Ionization energy vs. atomic number graph Chapter 55 Section Section 11 History History of of the the Chapter Periodic Table Table pages pages 133-137 ...
Document
... Albert Einstein (1879-1955) was born in Germany. Nothing in his early development suggested genius; even at the age of 9 he did not speak clearly, and his school principal replied, “it doesn’t matter; he’ll never make a success of anything.” When he was 10, Einstein entered the Luitpold Gymnaium (hi ...
... Albert Einstein (1879-1955) was born in Germany. Nothing in his early development suggested genius; even at the age of 9 he did not speak clearly, and his school principal replied, “it doesn’t matter; he’ll never make a success of anything.” When he was 10, Einstein entered the Luitpold Gymnaium (hi ...
Chapter 4 ppt(1)
... • are listed on the inside front cover of this text Learning Goal Given the name of an element, write its correct symbol; from the symbol, write the correct name. Chemistry: An Introduction to General, Organic, and Biological Chemistry, Twelfth Edition ...
... • are listed on the inside front cover of this text Learning Goal Given the name of an element, write its correct symbol; from the symbol, write the correct name. Chemistry: An Introduction to General, Organic, and Biological Chemistry, Twelfth Edition ...
Periodic Table Review File
... ____ 63. When determining the size of an atom by measuring the distance between bonded, identical, adjacent nuclei, the radius of an atom is a. equal to the distance between nuclei. b. one-half the distance between nuclei. c. twice the distance between nuclei. d. one-fourth the distance between nucl ...
... ____ 63. When determining the size of an atom by measuring the distance between bonded, identical, adjacent nuclei, the radius of an atom is a. equal to the distance between nuclei. b. one-half the distance between nuclei. c. twice the distance between nuclei. d. one-fourth the distance between nucl ...
Research Article - SciTech Publishers
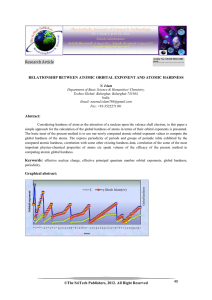
... It is significant to mention here that in order to evaluate the orbital exponent, we have used the Eq.(4) and the value of n* proposed by Slater 16 for n=1 to n=6 and for n=7, we have used the value of n*= 4.3 proposed by Ghosh and Biswas[25]. Now, the hardness refers to the resistance of the electr ...
... It is significant to mention here that in order to evaluate the orbital exponent, we have used the Eq.(4) and the value of n* proposed by Slater 16 for n=1 to n=6 and for n=7, we have used the value of n*= 4.3 proposed by Ghosh and Biswas[25]. Now, the hardness refers to the resistance of the electr ...
Chm 1
... ____ 59. In a row in the periodic table, as the atomic number increases, the atomic radius generally a. decreases. c. increases. b. remains constant. d. becomes immeasurable. ____ 60. Within a group of elements, as the atomic number increases, the atomic radius a. increases. b. remains approximatel ...
... ____ 59. In a row in the periodic table, as the atomic number increases, the atomic radius generally a. decreases. c. increases. b. remains constant. d. becomes immeasurable. ____ 60. Within a group of elements, as the atomic number increases, the atomic radius a. increases. b. remains approximatel ...
Chapter 4 - My Chemistry Site
... alkali metals compare with each other? They have the same number of valence electrons in their outer s orbital. 6. Why are the alkaline-earth metals less reactive than the alkali metals? Alkaline earth metals must lose 2 electrons instead of one to become stable. 7. In which groups of the periodic t ...
... alkali metals compare with each other? They have the same number of valence electrons in their outer s orbital. 6. Why are the alkaline-earth metals less reactive than the alkali metals? Alkaline earth metals must lose 2 electrons instead of one to become stable. 7. In which groups of the periodic t ...
Preview as PDF - Pearson Higher Education
... the aircraft’s wing. In fact, the majority of the plane is most likely made out of aluminum. Aluminum has several properties that make it suitable for airplane construction, but among the most important is its low density. Aluminum has a density of only 2.70 g>cm3. For comparison, iron’s density is ...
... the aircraft’s wing. In fact, the majority of the plane is most likely made out of aluminum. Aluminum has several properties that make it suitable for airplane construction, but among the most important is its low density. Aluminum has a density of only 2.70 g>cm3. For comparison, iron’s density is ...
Chapter 4
... • The transition metals constitute Groups 3 through 12 and are sometimes called the d-block elements because of their position in the periodic table. • A transition metal is one of the metals that can use the inner shell before using the outer shell to bond. • A transition metal may lose one, two, o ...
... • The transition metals constitute Groups 3 through 12 and are sometimes called the d-block elements because of their position in the periodic table. • A transition metal is one of the metals that can use the inner shell before using the outer shell to bond. • A transition metal may lose one, two, o ...
Snakes and Ladders Gaming Instructions
... 9. If you are on a spot other than at the bottom of a ladder or the top of a snake, then you must answer a moderately difficult Regular question in order to move forward on the board the correct number of spaces according to what you roll from the die. 10. Once a winner is declared, you have the opt ...
... 9. If you are on a spot other than at the bottom of a ladder or the top of a snake, then you must answer a moderately difficult Regular question in order to move forward on the board the correct number of spaces according to what you roll from the die. 10. Once a winner is declared, you have the opt ...
6.3 Periodic Trends
... 6.3 Periodic Trends > Trends in Atomic Size One way to think about atomic size is to look at the units that form when atoms of the same element are joined to one another. • These units are called molecules. • Because the atoms in each molecule shown below are identical, the distance between the nuc ...
... 6.3 Periodic Trends > Trends in Atomic Size One way to think about atomic size is to look at the units that form when atoms of the same element are joined to one another. • These units are called molecules. • Because the atoms in each molecule shown below are identical, the distance between the nuc ...
oxidation number
... • We predict nitrogen to have an oxidation number of -3. • N can have positive oxidation numbers such as +5. • However other positive oxidation numbers are possible as in nitrogen (I) oxide (laughing gas) N2O. Here nitrogen has an oxidation number of +1. ...
... • We predict nitrogen to have an oxidation number of -3. • N can have positive oxidation numbers such as +5. • However other positive oxidation numbers are possible as in nitrogen (I) oxide (laughing gas) N2O. Here nitrogen has an oxidation number of +1. ...
chem preap Periodic table practice test
... In the space provided, identify the period and block to which each of the following elements belongs. 35. Strontium: 1s22s22p63s23p63d104s24p65s2 In the space provided, write the ground-state valence electron configuration for each of the following elements. 36. Group 15, Period 3 In the space provi ...
... In the space provided, identify the period and block to which each of the following elements belongs. 35. Strontium: 1s22s22p63s23p63d104s24p65s2 In the space provided, write the ground-state valence electron configuration for each of the following elements. 36. Group 15, Period 3 In the space provi ...
chemistry chapter 4 powerpoint notes
... • The transition metals constitute Groups 3 through 12 and are sometimes called the d-block elements because of their position in the periodic table. • A transition metal is one of the metals that can use the inner shell before using the outer shell to bond. • A transition metal may lose one, two, o ...
... • The transition metals constitute Groups 3 through 12 and are sometimes called the d-block elements because of their position in the periodic table. • A transition metal is one of the metals that can use the inner shell before using the outer shell to bond. • A transition metal may lose one, two, o ...
Chapter 4 Ppt - s3.amazonaws.com
... • The transition metals constitute Groups 3 through 12 and are sometimes called the d-block elements because of their position in the periodic table. • A transition metal is one of the metals that can use the inner shell before using the outer shell to bond. • A transition metal may lose one, two, o ...
... • The transition metals constitute Groups 3 through 12 and are sometimes called the d-block elements because of their position in the periodic table. • A transition metal is one of the metals that can use the inner shell before using the outer shell to bond. • A transition metal may lose one, two, o ...
Although the weather changes from day to day, the weather you
... You are familiar with using a weather map to identify trends in the weather. For example, certain areas are typically warmer than other areas. What trends in the properties of elements can you identify with the help of the periodic table? You can identify trends in ...
... You are familiar with using a weather map to identify trends in the weather. For example, certain areas are typically warmer than other areas. What trends in the properties of elements can you identify with the help of the periodic table? You can identify trends in ...
oxidation number
... – However other than the noble gases which have an oxidation number of zero, and fluoride (F-) which is always a 1-, all other nonmetals could have positive oxidation numbers as well. ...
... – However other than the noble gases which have an oxidation number of zero, and fluoride (F-) which is always a 1-, all other nonmetals could have positive oxidation numbers as well. ...
6.3 Periodic Trends
... 6.3 Periodic Trends > Trends in Atomic Size One way to think about atomic size is to look at the units that form when atoms of the same element are joined to one another. • These units are called molecules. • Because the atoms in each molecule shown below are identical, the distance between the nuc ...
... 6.3 Periodic Trends > Trends in Atomic Size One way to think about atomic size is to look at the units that form when atoms of the same element are joined to one another. • These units are called molecules. • Because the atoms in each molecule shown below are identical, the distance between the nuc ...
6.3 Periodic Trends > Chapter 6
... 6.3 Periodic Trends > Trends in Atomic Size One way to think about atomic size is to look at the units that form when atoms of the same element are joined to one another. • These units are called molecules. • Because the atoms in each molecule shown below are identical, the distance between the nuc ...
... 6.3 Periodic Trends > Trends in Atomic Size One way to think about atomic size is to look at the units that form when atoms of the same element are joined to one another. • These units are called molecules. • Because the atoms in each molecule shown below are identical, the distance between the nuc ...
6_3 Periodic Trends2
... 6.3 Periodic Trends > Trends in Atomic Size One way to think about atomic size is to look at the units that form when atoms of the same element are joined to one another. • These units are called molecules. • Because the atoms in each molecule shown below are identical, the distance between the nuc ...
... 6.3 Periodic Trends > Trends in Atomic Size One way to think about atomic size is to look at the units that form when atoms of the same element are joined to one another. • These units are called molecules. • Because the atoms in each molecule shown below are identical, the distance between the nuc ...
Chapter 6 Section 6_3 Periodic Trends
... Ionic size tends to increase from top to bottom within a group. Generally, the size of cations and anions decreases from left to right across a period. In general, electronegativity values decrease from top to bottom within a group. For representative elements, the values tend to increase from left ...
... Ionic size tends to increase from top to bottom within a group. Generally, the size of cations and anions decreases from left to right across a period. In general, electronegativity values decrease from top to bottom within a group. For representative elements, the values tend to increase from left ...
Period 2 element
The period 2 elements are the chemical elements in the second row (or period) of the periodic table. The periodic table is laid out in rows to illustrate recurring (periodic) trends in the chemical behavior of the elements as their atomic number increases; a new row is started when chemical behavior begins to repeat, creating columns of elements with similar properties.The second period contains the elements lithium, beryllium, boron, carbon, nitrogen, oxygen, fluorine, and neon. This situation can be explained by modern theories of atomic structure. In a quantum mechanical description of atomic structure, this period corresponds to the filling of the 2s and 2p orbitals. Period 2 elements obey the octet rule in that they need eight electrons to complete their valence shell. The maximum number of electrons that these elements can accommodate is ten, two in the 1s orbital, two in the 2s orbital and six in the 2p orbital. All of the elements in the period can form diatomic molecules except beryllium and neon.