Topic 7 - Lloyd Crosby
... (1) Each orbital (specific ml ) can hold no more than two electrons. (2) Two electrons can occupy the same orbital only if they have opposite spins (different ms). 3. Hund’s Rule a. Definition The most stable arrangement of electrons in subshells is the one with the greatest number of parallel spins ...
... (1) Each orbital (specific ml ) can hold no more than two electrons. (2) Two electrons can occupy the same orbital only if they have opposite spins (different ms). 3. Hund’s Rule a. Definition The most stable arrangement of electrons in subshells is the one with the greatest number of parallel spins ...
Document
... • With carbon, you must apply Hund’s rule. In other words, carbon’s sixth electron must go into the next unoccupied 2p orbital. (Experimental evidence confirms that the spin of this sixth electron is, in fact, the same direction as the fifth electron.) • With oxygen, as with helium’s 1s orbital and ...
... • With carbon, you must apply Hund’s rule. In other words, carbon’s sixth electron must go into the next unoccupied 2p orbital. (Experimental evidence confirms that the spin of this sixth electron is, in fact, the same direction as the fifth electron.) • With oxygen, as with helium’s 1s orbital and ...
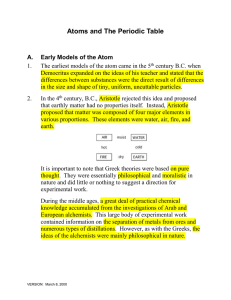
Atoms and The Periodic Table
... predicted the pattern of energies that can be produced by a hydrogen atom. In order to derive his equation, Bohr suggested that electrons could only exist in fixed energy or “quantized” energy orbits. Since electrons could only exist in these orbits, it was not possible for them to spiral into the n ...
... predicted the pattern of energies that can be produced by a hydrogen atom. In order to derive his equation, Bohr suggested that electrons could only exist in fixed energy or “quantized” energy orbits. Since electrons could only exist in these orbits, it was not possible for them to spiral into the n ...
Unit VIII Atoms… - VernonScienceLSA
... predicted the pattern of energies that can be produced by a hydrogen atom. In order to derive his equation, Bohr suggested that electrons could only exist in fixed energy or “quantized” energy orbits. Since electrons could only exist in these orbits, it was not possible for them to spiral into the n ...
... predicted the pattern of energies that can be produced by a hydrogen atom. In order to derive his equation, Bohr suggested that electrons could only exist in fixed energy or “quantized” energy orbits. Since electrons could only exist in these orbits, it was not possible for them to spiral into the n ...
Chapter 2: The Structure of the Atom and the Periodic
... D) A chlorine atom needs one electron to obtain an octet in its outermost energy level. E) A chlorine atom has 17 protons. Ans: C 30. In nature, the element neon exists as three different isotopes: Ne-20, Ne-21, and Ne-22. Which isotope would be the most abundant in a sample of neon? A) Ne-20 B) Ne- ...
... D) A chlorine atom needs one electron to obtain an octet in its outermost energy level. E) A chlorine atom has 17 protons. Ans: C 30. In nature, the element neon exists as three different isotopes: Ne-20, Ne-21, and Ne-22. Which isotope would be the most abundant in a sample of neon? A) Ne-20 B) Ne- ...
Chapter 2: The Structure of the Atom and the Periodic
... D) A chlorine atom needs one electron to obtain an octet in its outermost energy level. E) A chlorine atom has 17 protons. Ans: C 30. In nature, the element neon exists as three different isotopes: Ne-20, Ne-21, and Ne-22. Which isotope would be the most abundant in a sample of neon? A) Ne-20 B) Ne- ...
... D) A chlorine atom needs one electron to obtain an octet in its outermost energy level. E) A chlorine atom has 17 protons. Ans: C 30. In nature, the element neon exists as three different isotopes: Ne-20, Ne-21, and Ne-22. Which isotope would be the most abundant in a sample of neon? A) Ne-20 B) Ne- ...
Electronic Structure and Periodic Properties
... 3d and 4s orbitals half filled to minimize electron repulsions. ≡ Hund’s Rule Similarly complete 3d orbitals are favorable eg 29Cu. The energy of 3d falls below that of 4s because of the increased nuclear charge. Electronic configuration of 29Cu = [Ar]4s13d10 Cu and Cr belong to the first series of ...
... 3d and 4s orbitals half filled to minimize electron repulsions. ≡ Hund’s Rule Similarly complete 3d orbitals are favorable eg 29Cu. The energy of 3d falls below that of 4s because of the increased nuclear charge. Electronic configuration of 29Cu = [Ar]4s13d10 Cu and Cr belong to the first series of ...
LETTER TO THE EDITOR: “Cheese or Flu?” William B. Jensen
... intending to communicate it to the Russian Chemical Society ... at the meeting on March 6 [i.e. March 18]. Illness prevented him from attending, and the paper was read, at his request, by my father, Nikolai Alekansdorvic Menschutkin, at the time Professor of analytical chemistry at the University of ...
... intending to communicate it to the Russian Chemical Society ... at the meeting on March 6 [i.e. March 18]. Illness prevented him from attending, and the paper was read, at his request, by my father, Nikolai Alekansdorvic Menschutkin, at the time Professor of analytical chemistry at the University of ...
Periods and Blocks of the Periodic Table
... • Element A has a very low ionization energy, which means that atoms of A lose electrons easily. • Element A is most likely to be an s-block metal because ionization energies increase across the periods. • Element B has a very high ionization energy which means that atoms of B have difficulty losing ...
... • Element A has a very low ionization energy, which means that atoms of A lose electrons easily. • Element A is most likely to be an s-block metal because ionization energies increase across the periods. • Element B has a very high ionization energy which means that atoms of B have difficulty losing ...
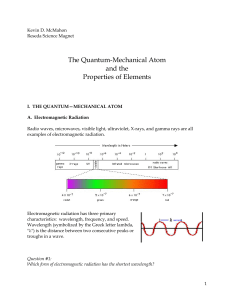
Kevin D - CSUN.edu
... When l = 2 = d, there are five values of ml: -2, -1, 0, 1, 2. The sublevel d has five orbitals. There are no d orbitals when n = 1 and n = 2. When l = 3= f, there are seven values of ml: -3, -2, -1, 0, 1, 2, 3. The sublevel f has seven orbitals. There are no f orbitals when n = 1, 2, or 3. Electrons ...
... When l = 2 = d, there are five values of ml: -2, -1, 0, 1, 2. The sublevel d has five orbitals. There are no d orbitals when n = 1 and n = 2. When l = 3= f, there are seven values of ml: -3, -2, -1, 0, 1, 2, 3. The sublevel f has seven orbitals. There are no f orbitals when n = 1, 2, or 3. Electrons ...
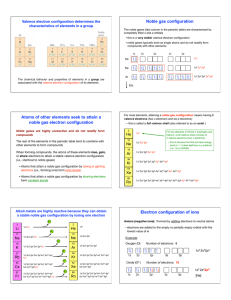
Noble gas configuration Atoms of other elements seek to attain a
... The chemical behavior and properties of elements in a group are associated with the valence electron configuration of its elements ...
... The chemical behavior and properties of elements in a group are associated with the valence electron configuration of its elements ...
Periodic Table Review
... They have 8 valence electrons They have a full outermost electron shell ...
... They have 8 valence electrons They have a full outermost electron shell ...
Periodic Trends in Atomic Properties
... 1. It is the number and type of valence electrons that primarily determine an atom’s chemistry. 2. Electron configurations can be determined from the organization of the periodic table. 3. Certain groups in the periodic table have special names. ...
... 1. It is the number and type of valence electrons that primarily determine an atom’s chemistry. 2. Electron configurations can be determined from the organization of the periodic table. 3. Certain groups in the periodic table have special names. ...
Chapter 7 Periodic Properties of the Elements
... under the curves, which represents the probability of finding an electron at that particular distance, there is way more area under the 2s curve farther away from the nucleus. ...
... under the curves, which represents the probability of finding an electron at that particular distance, there is way more area under the 2s curve farther away from the nucleus. ...
ATOMIC ELECTRON CONFIGURATIONS AND PERIODICITY
... largest radius. Since Mg2+ has the lowest, it must have the smallest radius. The rest can be ranked by ratio. ...
... largest radius. Since Mg2+ has the lowest, it must have the smallest radius. The rest can be ranked by ratio. ...
Chapter 5
... • The number of the highest occupied energy level is 5, so the element is in the fifth period. • There are five electrons in the d sublevel, which means that it is incompletely filled. The d sublevel can hold 10 electrons. Therefore, the element is in the d block. • For d-block elements, the number ...
... • The number of the highest occupied energy level is 5, so the element is in the fifth period. • There are five electrons in the d sublevel, which means that it is incompletely filled. The d sublevel can hold 10 electrons. Therefore, the element is in the d block. • For d-block elements, the number ...
Periods and Blocks of the Periodic Table
... • The number of the highest occupied energy level is 5, so the element is in the fifth period. • There are five electrons in the d sublevel, which means that it is incompletely filled. The d sublevel can hold 10 electrons. Therefore, the element is in the d block. • For d-block elements, the number ...
... • The number of the highest occupied energy level is 5, so the element is in the fifth period. • There are five electrons in the d sublevel, which means that it is incompletely filled. The d sublevel can hold 10 electrons. Therefore, the element is in the d block. • For d-block elements, the number ...
Chapter 7 Electron Configuration and the Periodic Table
... – Have single valence electron – Properties differ – Group 1B much less reactive than 1A – High IE of 1B - incomplete shielding of nucleus by inner “d” and outer “s” electrons of 1B strongly attracted to nucleus – 1B metals often found elemental in nature (coinage metals) ...
... – Have single valence electron – Properties differ – Group 1B much less reactive than 1A – High IE of 1B - incomplete shielding of nucleus by inner “d” and outer “s” electrons of 1B strongly attracted to nucleus – 1B metals often found elemental in nature (coinage metals) ...
Ch7temp
... – Have single valence electron – Properties differ – Group 1B much less reactive than 1A – High IE of 1B - incomplete shielding of nucleus by inner “d” and outer “s” electrons of 1B strongly attracted to nucleus – 1B metals often found elemental in nature (coinage metals) ...
... – Have single valence electron – Properties differ – Group 1B much less reactive than 1A – High IE of 1B - incomplete shielding of nucleus by inner “d” and outer “s” electrons of 1B strongly attracted to nucleus – 1B metals often found elemental in nature (coinage metals) ...
ch 3 classification of elements and periodic properties
... Most of them are non-metals, highly electronegative, form www.chemzblog.wordpress.com.....Zaid acidic oxides. Mansuri 9824662116 ...
... Most of them are non-metals, highly electronegative, form www.chemzblog.wordpress.com.....Zaid acidic oxides. Mansuri 9824662116 ...
1 Periodic Properties of the Elements Chapter 7 CHEMA1301 2014
... Let’s look at the Na atom to see what to expect for the magnitude of Zeff. Sodium has the electron configuration [Ne]3s1 . The nuclear charge is (Z =11+), and there are 10 core electrons (1s22s22p6). We therefore expect S to equal 10 and the 3s electron to experience an effective nuclear charge of Z ...
... Let’s look at the Na atom to see what to expect for the magnitude of Zeff. Sodium has the electron configuration [Ne]3s1 . The nuclear charge is (Z =11+), and there are 10 core electrons (1s22s22p6). We therefore expect S to equal 10 and the 3s electron to experience an effective nuclear charge of Z ...
Unit 1-4-Periodic Trends - Free WonderKids-e
... Important exceptions of the above rules include the noble gases, lanthanides, and actinides. The noble gases possess a complete valence shell and do not usually attract electrons. The lanthanides and actinides possess a more complicated chemistry that does not generally follow any trends. Therefore, ...
... Important exceptions of the above rules include the noble gases, lanthanides, and actinides. The noble gases possess a complete valence shell and do not usually attract electrons. The lanthanides and actinides possess a more complicated chemistry that does not generally follow any trends. Therefore, ...
Chapter 6: The Periodic Table and Periodic Law
... who is shown in Figure 6-2, proposed an organization scheme for the elements. Newlands noticed that when the elements were arranged by increasing atomic mass, their properties repeated every eighth element. In other words, the first and eighth elements had similar properties, the second and ninth el ...
... who is shown in Figure 6-2, proposed an organization scheme for the elements. Newlands noticed that when the elements were arranged by increasing atomic mass, their properties repeated every eighth element. In other words, the first and eighth elements had similar properties, the second and ninth el ...
Period 2 element
The period 2 elements are the chemical elements in the second row (or period) of the periodic table. The periodic table is laid out in rows to illustrate recurring (periodic) trends in the chemical behavior of the elements as their atomic number increases; a new row is started when chemical behavior begins to repeat, creating columns of elements with similar properties.The second period contains the elements lithium, beryllium, boron, carbon, nitrogen, oxygen, fluorine, and neon. This situation can be explained by modern theories of atomic structure. In a quantum mechanical description of atomic structure, this period corresponds to the filling of the 2s and 2p orbitals. Period 2 elements obey the octet rule in that they need eight electrons to complete their valence shell. The maximum number of electrons that these elements can accommodate is ten, two in the 1s orbital, two in the 2s orbital and six in the 2p orbital. All of the elements in the period can form diatomic molecules except beryllium and neon.