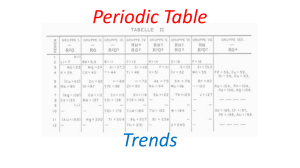
MS Word Printable
... 5. Find potassium. What is the atomic #? ______ atomic mass? ______ # of neutrons? _____ 6. In an electrically neutral carbon atom, how many protons are present? ____ how many electrons? _____ 7. The elements in the far right column, group 8 or VIII, are considered stable in terms of bonding. Name t ...
... 5. Find potassium. What is the atomic #? ______ atomic mass? ______ # of neutrons? _____ 6. In an electrically neutral carbon atom, how many protons are present? ____ how many electrons? _____ 7. The elements in the far right column, group 8 or VIII, are considered stable in terms of bonding. Name t ...
Name: Date: _____ Pd: _____ Chemistry, PERIODIC TABLE Spring
... Silver-plated utensils were popular before stainless steel became widely used to make eating utensils. Silver tarnishes when it comes in contact with hydrogen sulfide, , which is found in the air and in some foods. However, stainless steel does not tarnish when it comes in contact with hydrogen sulf ...
... Silver-plated utensils were popular before stainless steel became widely used to make eating utensils. Silver tarnishes when it comes in contact with hydrogen sulfide, , which is found in the air and in some foods. However, stainless steel does not tarnish when it comes in contact with hydrogen sulf ...
Anticipation Guide Before After The periodic table is a collection of
... The atomic number is the number of protons in the nucleus. The atomic number is written below the element on the Periodic Table. The atomic mass is the sum of protons and electrons in the nucleus. The atomic mass is written above the element on the Periodic Table. Atomic mass can be located mostly i ...
... The atomic number is the number of protons in the nucleus. The atomic number is written below the element on the Periodic Table. The atomic mass is the sum of protons and electrons in the nucleus. The atomic mass is written above the element on the Periodic Table. Atomic mass can be located mostly i ...
Elements and the Periodic Table
... 21. Would grom (Gr) or bart (B) have properties that are more similar to those of the element twee (Tw)? Explain why. ________________________________________________________________________ ________________________________________________________________________ 22. Based on its atomic mass and its ...
... 21. Would grom (Gr) or bart (B) have properties that are more similar to those of the element twee (Tw)? Explain why. ________________________________________________________________________ ________________________________________________________________________ 22. Based on its atomic mass and its ...
Elements and the Periodic Table Practice Test
... 9. There exists several _____________ of argon, such as Ar-36, Ar-38, and Ar-40. 10.Yet the mass for argon on the periodic table is 39.948 atomic mass units and not 36, 38, or 40. Explain why. ...
... 9. There exists several _____________ of argon, such as Ar-36, Ar-38, and Ar-40. 10.Yet the mass for argon on the periodic table is 39.948 atomic mass units and not 36, 38, or 40. Explain why. ...
2- Periodic Trends
... •Elements in groups have similar properties A family is a group with a specific name: Family names and locations need to be memorized GROUP ...
... •Elements in groups have similar properties A family is a group with a specific name: Family names and locations need to be memorized GROUP ...
Periodic Table
... Elements in the same group have similar physical and chemical properties Sometimes groups are called families ...
... Elements in the same group have similar physical and chemical properties Sometimes groups are called families ...
Page|1 - askIITians
... 27. Would you expect the second electron gain enthalpy of O as positive, more negative or less negative than the first? Justify your answer. ...
... 27. Would you expect the second electron gain enthalpy of O as positive, more negative or less negative than the first? Justify your answer. ...
The Periodic Table Worksheet
... 11. There are two rows of elements on the bottom of the table. These elements are the rare earth metals. What is the name given to each of these rows of elements? ___________________ ___________________ 12. Name two elements in group VIIA. ___________________ ___________________ ...
... 11. There are two rows of elements on the bottom of the table. These elements are the rare earth metals. What is the name given to each of these rows of elements? ___________________ ___________________ 12. Name two elements in group VIIA. ___________________ ___________________ ...
Reinforcing Key Concepts
... radioactivity by the time it takes for one-half of a sample of atoms to change identity. For example, lead-214 has a half-life of 27 minutes. If you started with 500 grams of this isotope, how many grams would you have after 54 minutes? ...
... radioactivity by the time it takes for one-half of a sample of atoms to change identity. For example, lead-214 has a half-life of 27 minutes. If you started with 500 grams of this isotope, how many grams would you have after 54 minutes? ...
Define the following: Electronegativity
... 17. The atomic number of an element is the total number of which particles in the nucleus? Protons and also electrons if electrically neutral 18. What is the electron configuration of the noble gases? End with s2p6 19. Which subatomic particle plays the greatest part in determining the properties of ...
... 17. The atomic number of an element is the total number of which particles in the nucleus? Protons and also electrons if electrically neutral 18. What is the electron configuration of the noble gases? End with s2p6 19. Which subatomic particle plays the greatest part in determining the properties of ...
The Periodic Table Worksheet
... 4. a) What is the link between the group to which an element belongs in the periodic table and the number of electrons in an atom of the element’s outer shell? ...
... 4. a) What is the link between the group to which an element belongs in the periodic table and the number of electrons in an atom of the element’s outer shell? ...
Trends in Atomic Radii – Visualization Activity
... can be removed from or attracted to the atom, and that depends on their distance from the attractive force of the __________________. The further away electrons are from the nucleus, the ________________ easily they can be removed and the ___________________ reactive the metal element is. For n ...
... can be removed from or attracted to the atom, and that depends on their distance from the attractive force of the __________________. The further away electrons are from the nucleus, the ________________ easily they can be removed and the ___________________ reactive the metal element is. For n ...
Me, Myself, I, Chlorine BY: Ethan. BP:2
... Me, Myself, I, Chlorine BY: Ethan. B P:2 It started in a lab in Sweden. Chlorine was first produced by Carl Wilhelm Scheele, a Swedish chemist, when he combined the mineral pyrolusite (MnO2) with hydrochloric acid (HCl) in 1774. That is how i was born. (Beautiful right?) MY ...
... Me, Myself, I, Chlorine BY: Ethan. B P:2 It started in a lab in Sweden. Chlorine was first produced by Carl Wilhelm Scheele, a Swedish chemist, when he combined the mineral pyrolusite (MnO2) with hydrochloric acid (HCl) in 1774. That is how i was born. (Beautiful right?) MY ...
Int. Sci. 9 Modern Periodic Table Powerpoint
... – Members of a group in the periodic table have similar chemical properties Known as Periodic Law!!! ...
... – Members of a group in the periodic table have similar chemical properties Known as Periodic Law!!! ...
Chemical-Periodicity
... • Atomic size generally increases as you move down a group. • Atomic size generally decreases as you move from left to right across a period. Largest atoms are towards the bottom and to the left of the periodic table. ...
... • Atomic size generally increases as you move down a group. • Atomic size generally decreases as you move from left to right across a period. Largest atoms are towards the bottom and to the left of the periodic table. ...
CHMB homework Name © Van Der Sluys, 2004 Periodic Table 1
... 1. Where are the most active metals? _________________________________ 2. Where are the most active nonmetals? ______________________________ 3. As you go from left to right across a period, the atomic size (increases/decreases). 4. As you travel down a group, the atomic size (decreases/increases). ...
... 1. Where are the most active metals? _________________________________ 2. Where are the most active nonmetals? ______________________________ 3. As you go from left to right across a period, the atomic size (increases/decreases). 4. As you travel down a group, the atomic size (decreases/increases). ...
Alchemy Unit Concepts Review
... Molecular covalent Ionic c. Zinc, Zn (s) d. Phosphorus trifluoride, PF3 (g) Metallic Molecular Covalent e. C(s), graphite Network covalent 5. a) What is an isotope? Isotopes are atoms of an element with different number of neutrons. b) Predict the different isotopes for the following elements and wr ...
... Molecular covalent Ionic c. Zinc, Zn (s) d. Phosphorus trifluoride, PF3 (g) Metallic Molecular Covalent e. C(s), graphite Network covalent 5. a) What is an isotope? Isotopes are atoms of an element with different number of neutrons. b) Predict the different isotopes for the following elements and wr ...
Periodic Table – an arrangement of the elements in order of their
... Alkali metal – an element in Group 1a of the periodic table. Alkaline earth metal – an element in Group 2a of the periodic table. Halogen – a reactive nonmetallic element in Group 7a of the periodic table. Noble gas – an inactive element in Group 8a of the periodic table. Metal – an element that typ ...
... Alkali metal – an element in Group 1a of the periodic table. Alkaline earth metal – an element in Group 2a of the periodic table. Halogen – a reactive nonmetallic element in Group 7a of the periodic table. Noble gas – an inactive element in Group 8a of the periodic table. Metal – an element that typ ...
Textbook Questions - Teach-n-Learn-Chem
... 30. Cations are always __________ than the atoms from which they form; anions are always _________. 31. Why are the electrons drawn closer to the nucleus when a sodium atom loses an electron? ...
... 30. Cations are always __________ than the atoms from which they form; anions are always _________. 31. Why are the electrons drawn closer to the nucleus when a sodium atom loses an electron? ...
Section 5.1 Review
... (b) the physical and chemical properties of an element are functions of its atomic number. (c) electrons exhibit properties of both particles and waves. (d) the chemical properties of elements can be grouped according to periodicity, but physical properties cannot. 4. _____ The discovery of the nobl ...
... (b) the physical and chemical properties of an element are functions of its atomic number. (c) electrons exhibit properties of both particles and waves. (d) the chemical properties of elements can be grouped according to periodicity, but physical properties cannot. 4. _____ The discovery of the nobl ...
Notes Chapter 5 “The Periodic Law” Section 1 Dmitri Mendeleev
... Today periodic table is arranged according to atomic number and properties. 1894 Strutt and Ramsay discovered Argon the first of the noble gases. The Lanthanide – All end in 4f, numbers 58-71, period 6. Actinides – all end in 5f, number 90-103. Belongs in period 7. **to save space the lanthanides an ...
... Today periodic table is arranged according to atomic number and properties. 1894 Strutt and Ramsay discovered Argon the first of the noble gases. The Lanthanide – All end in 4f, numbers 58-71, period 6. Actinides – all end in 5f, number 90-103. Belongs in period 7. **to save space the lanthanides an ...
C3 The Periodic Table
... ordered (based on patterns of behaviour, then on mass) and made little sense. • In 1964 Newlands attempted to put it in a sensible order by mass and saw similarities between every 8th element . • Mendeleev ordered the elements based on their atomic weights and arranged them so there was a pattern. • ...
... ordered (based on patterns of behaviour, then on mass) and made little sense. • In 1964 Newlands attempted to put it in a sensible order by mass and saw similarities between every 8th element . • Mendeleev ordered the elements based on their atomic weights and arranged them so there was a pattern. • ...