ATOMS ELEMENTS PERIODIC TABLE MOLECULES COMPOUNDS
... • What is the difference between a compound and a molecule? • A molecule is formed when two or more atoms join together chemically. A compound is a molecule that contains at least two different elements. All compounds are molecules but not all molecules are compounds. • Molecular hydrogen (H2), mole ...
... • What is the difference between a compound and a molecule? • A molecule is formed when two or more atoms join together chemically. A compound is a molecule that contains at least two different elements. All compounds are molecules but not all molecules are compounds. • Molecular hydrogen (H2), mole ...
Science Review Sheet: Periodic Table Test Name: ______ Study
... 4. What do valence electrons tell us about an atom? ...
... 4. What do valence electrons tell us about an atom? ...
Atomic Structure and The Periodic Table
... Key Learning: Atomic structure determines the properties of elements and their positions on the periodic table. Unit Essential Question: How does the atomic structure determine the properties of elements and their positions on the periodic table? ...
... Key Learning: Atomic structure determines the properties of elements and their positions on the periodic table. Unit Essential Question: How does the atomic structure determine the properties of elements and their positions on the periodic table? ...
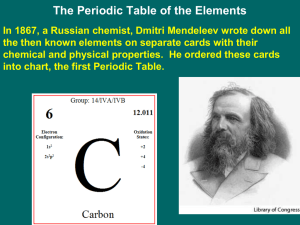
2.5-The Periodic Table
... take its place. He predicted the properties of the “unknown” elements, and within the next sixteen years those gaps were filled in with newly discovered elements that matched precisely with Mendeleev’s predictions! ...
... take its place. He predicted the properties of the “unknown” elements, and within the next sixteen years those gaps were filled in with newly discovered elements that matched precisely with Mendeleev’s predictions! ...
CP CHEMISTRY STUDY GUIDE
... PA Academic Standards - Science & Technology 3.1.10.C. Apply and assess patterns as repeated processes or recurring elements in science and technology. 3.4.10/12.A. Explain and apply concepts about the structure and properties of matter. ...
... PA Academic Standards - Science & Technology 3.1.10.C. Apply and assess patterns as repeated processes or recurring elements in science and technology. 3.4.10/12.A. Explain and apply concepts about the structure and properties of matter. ...
Unit 4 Review - Davis
... The atomic radius is a measure of the size of an atom. Modern Periodic Law – The properties of the elements are a periodic function of their atomic numbers. The statement that the physical and chemical properties of the elements repeat in a regular pattern when they are arranged in order of increasi ...
... The atomic radius is a measure of the size of an atom. Modern Periodic Law – The properties of the elements are a periodic function of their atomic numbers. The statement that the physical and chemical properties of the elements repeat in a regular pattern when they are arranged in order of increasi ...
Periodicity review handout
... ____________________ An element with a large negative electron affinity is most likely to form a positive ion, a negative ion, or a neutral ion? ...
... ____________________ An element with a large negative electron affinity is most likely to form a positive ion, a negative ion, or a neutral ion? ...
Chapter 12 The Periodic Table
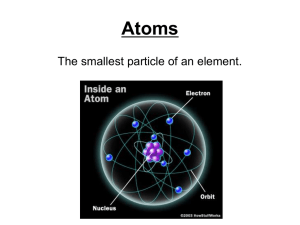
... Remember, the atomic number is the number of protons all atoms of that element have in their nuclei. If the atom is neutral, it will have the same number of electrons as protons. ...
... Remember, the atomic number is the number of protons all atoms of that element have in their nuclei. If the atom is neutral, it will have the same number of electrons as protons. ...
2.2 Periodic Chart
... distinctive colours (Ne is reddish) when electricity is passed through them. Their ion charges of zero indicate that they do not form charged ions. ...
... distinctive colours (Ne is reddish) when electricity is passed through them. Their ion charges of zero indicate that they do not form charged ions. ...
The Periodic Table
... • Elements that behave in a similar way are arranged in vertical columns called groups. • A group is a vertical column of elements that all have the same number of electrons in the outer shell. • All elements in a group behave in a similar ...
... • Elements that behave in a similar way are arranged in vertical columns called groups. • A group is a vertical column of elements that all have the same number of electrons in the outer shell. • All elements in a group behave in a similar ...
The Periodic Table assignment
... Name__________________________________ period _____ date assigned_____________ date due ______________ date returned _____________ ...
... Name__________________________________ period _____ date assigned_____________ date due ______________ date returned _____________ ...
2012 chapter 4 study guide
... The organization of the periodic table is based on the properties of the elements and reflects the structure of atoms. As a basis for understanding this concept: 7 a. Students know how to identify regions corresponding to metals, nonmetals, and inert gases. Be able to 7. tell who Mendeleev was and h ...
... The organization of the periodic table is based on the properties of the elements and reflects the structure of atoms. As a basis for understanding this concept: 7 a. Students know how to identify regions corresponding to metals, nonmetals, and inert gases. Be able to 7. tell who Mendeleev was and h ...
Atoms and Periodic Table
... Alkali Metals Elements with only one electron in the outermost energy level of their atoms; located in Group 1 on the Periodic Table; not stable and tend to lose their one electron easily. ...
... Alkali Metals Elements with only one electron in the outermost energy level of their atoms; located in Group 1 on the Periodic Table; not stable and tend to lose their one electron easily. ...
ELEMENTS and THEIR PROPERTIES
... Transition Elements- they often occur in nature as uncombined elements • Typically form colored compounds- chromium found in rubies and emeralds • Iron triad- iron, cobalt, and nickel iron- most widely used of all metals and main ingredient in steel; abundant in Earth’s crust Cobalt and Nickel- use ...
... Transition Elements- they often occur in nature as uncombined elements • Typically form colored compounds- chromium found in rubies and emeralds • Iron triad- iron, cobalt, and nickel iron- most widely used of all metals and main ingredient in steel; abundant in Earth’s crust Cobalt and Nickel- use ...
Chapter 6 Periodic law- states that when the elements are arranged
... Periodic law- states that when the elements are arranged by increasing atomic number, there is a periodic repetition of their chemical and physical properties Group- A vertical column of elements in the periodic table; also called a family Period- A horizontal row of elements in the modern periodic ...
... Periodic law- states that when the elements are arranged by increasing atomic number, there is a periodic repetition of their chemical and physical properties Group- A vertical column of elements in the periodic table; also called a family Period- A horizontal row of elements in the modern periodic ...
Name Period
... 2. What category of element (metal, non-metal, metalloid) is most common in the periodic table?______________ Where is that category found in the periodic table? __________________________________________________________ Vocabulary Write the correct term from the words below to complete each sentenc ...
... 2. What category of element (metal, non-metal, metalloid) is most common in the periodic table?______________ Where is that category found in the periodic table? __________________________________________________________ Vocabulary Write the correct term from the words below to complete each sentenc ...
Periodic Table Notes The Periodic Table Use Resource #1
... Periodic Table Notes The Periodic Table Use Resource #1 to complete this section. Define and describe: period – ...
... Periodic Table Notes The Periodic Table Use Resource #1 to complete this section. Define and describe: period – ...
Periodic Table cloze activity.
... atom, atomic number, column, element, gold, inert, Mendeleev, metals, nonmetals, periodic, properties, symbol All matter is composed of various elements. An _________________ is a form of matter that is composed of a single type of _________________. In 1869, Dmitri _________________ created the ___ ...
... atom, atomic number, column, element, gold, inert, Mendeleev, metals, nonmetals, periodic, properties, symbol All matter is composed of various elements. An _________________ is a form of matter that is composed of a single type of _________________. In 1869, Dmitri _________________ created the ___ ...
Colored Period Table
... The vertical columns of the periodic table (there are 18) are called groups or families. Elements in the same group or family have similar but not identical characteristics. You will learn more about the 18 groups in a later section. You can know properties of a certain element by knowing which grou ...
... The vertical columns of the periodic table (there are 18) are called groups or families. Elements in the same group or family have similar but not identical characteristics. You will learn more about the 18 groups in a later section. You can know properties of a certain element by knowing which grou ...
File
... 18. The elements on the periodic table are arranged by increasing _____________ ________________. (hint: what number continually gets bigger as the table proceeds?) 19. Define what a group is on the periodic table. 20. Define what a period is on the periodic table. 21. Moving from left to right acro ...
... 18. The elements on the periodic table are arranged by increasing _____________ ________________. (hint: what number continually gets bigger as the table proceeds?) 19. Define what a group is on the periodic table. 20. Define what a period is on the periodic table. 21. Moving from left to right acro ...
Chemistry Study Guide - Atomic structure and the Periodic Table 2010
... 3. Each of the more than 100 elements of matter has distinct properties and a distinct atomic structure. All forms of matter are composed of one or more of the elements. As a basis for understanding this concept: a. What is the structure of the atom and how are protons, neutrons, and electrons arran ...
... 3. Each of the more than 100 elements of matter has distinct properties and a distinct atomic structure. All forms of matter are composed of one or more of the elements. As a basis for understanding this concept: a. What is the structure of the atom and how are protons, neutrons, and electrons arran ...
Periodic Table Cloze - Science
... _________________ is a form of matter that is composed of a single type of _________________. In 1869, Dmitri _________________ created the Calcium: an element on the periodic table with atomic number 20. ...
... _________________ is a form of matter that is composed of a single type of _________________. In 1869, Dmitri _________________ created the Calcium: an element on the periodic table with atomic number 20. ...
The Periodic Table
... pattern to them and organized them as such. Properties are periodic meaning they had a repeating pattern ...
... pattern to them and organized them as such. Properties are periodic meaning they had a repeating pattern ...