noble gases
... certain elements even before they were discovered! Ex. Gallium was predicted in 1871 and not actually discovered until 1875. ...
... certain elements even before they were discovered! Ex. Gallium was predicted in 1871 and not actually discovered until 1875. ...
Name Periodic Table Assignment Directions: Using your text (pgs
... How are the elements on the periodic table arranged? What are the vertical columns on the periodic table called? What properties do these vertical columns have? What are the horizontal rows called? What properties do these horizontal rows have? Four groups on the periodic table have specific names. ...
... How are the elements on the periodic table arranged? What are the vertical columns on the periodic table called? What properties do these vertical columns have? What are the horizontal rows called? What properties do these horizontal rows have? Four groups on the periodic table have specific names. ...
Name Chemistry Midterm Review Chapter # 2
... 6. What are the 4 types of sub orbitals? How many “rooms” does each have? How many electrons can each of the 4 suborbitals hold? (make a chart) ...
... 6. What are the 4 types of sub orbitals? How many “rooms” does each have? How many electrons can each of the 4 suborbitals hold? (make a chart) ...
2.2 The Periodic table and Chemical Properties
... • Know how the elements are listed in rows by increasing order of Atomic number • Rows are arranged in such a way that elements with similar properties line up in vertical columns • Each element in the table is recorded using its name, symbol, atomic number, atomic mass, and common ion charges ...
... • Know how the elements are listed in rows by increasing order of Atomic number • Rows are arranged in such a way that elements with similar properties line up in vertical columns • Each element in the table is recorded using its name, symbol, atomic number, atomic mass, and common ion charges ...
The Periodic Table - Mr Linseman`s wiki
... Alkali metals: shiny, silvery metals that react easily, form compounds that are soluble in water (group 1) Alkaline earth metals: shiny, silvery metals, form compounds that are often insoluble in water (group 2) Halogens: poisonous, react readily with alkali metals (group 17) Noble gases: do ...
... Alkali metals: shiny, silvery metals that react easily, form compounds that are soluble in water (group 1) Alkaline earth metals: shiny, silvery metals, form compounds that are often insoluble in water (group 2) Halogens: poisonous, react readily with alkali metals (group 17) Noble gases: do ...
OBETA CHINONSO FAVOUR. 16/SCI14/018. GEOLOGY. CHM 221
... I. Electrolysis of their molten chlorides for metals like sodium, magnesium, calcium and potassium. ii. Reduction of oxides using carbons for metals like zinc, iron, tin, lead and copper. iii. Roasting of the ore by heating alone for metals like mercury, silver, gold and platinum. ...
... I. Electrolysis of their molten chlorides for metals like sodium, magnesium, calcium and potassium. ii. Reduction of oxides using carbons for metals like zinc, iron, tin, lead and copper. iii. Roasting of the ore by heating alone for metals like mercury, silver, gold and platinum. ...
Standard EPS Shell Presentation
... Remember, the atomic number is the number of protons all atoms of that element have in their nuclei. If the atom is neutral, it will have the same number of electrons as protons. ...
... Remember, the atomic number is the number of protons all atoms of that element have in their nuclei. If the atom is neutral, it will have the same number of electrons as protons. ...
Who`s in this family?
... up processes in the human body • It combines with many other elements to form useful compounds such as, milk of magnesia & Epsom salts ...
... up processes in the human body • It combines with many other elements to form useful compounds such as, milk of magnesia & Epsom salts ...
Structure of Atoms/Periodic Table Review 1. Shade in location of the
... 20. Why are atoms neutral? They have the _________ __________ of protons and neutrons. 22. What are the limitations of the Bohr model? ____________ do not orbit the nucleus like planets orbit the sun. The ___________ does not represent the actual size of an atom. 23. What determines an element’s ide ...
... 20. Why are atoms neutral? They have the _________ __________ of protons and neutrons. 22. What are the limitations of the Bohr model? ____________ do not orbit the nucleus like planets orbit the sun. The ___________ does not represent the actual size of an atom. 23. What determines an element’s ide ...
Study Guide - Chapter 12 Quiz
... C. most are solid at room temperature Nonmetals A. found to the right of the zigzag line B. most have an almost complete set of electrons in the outer level C. More than half are gases at room temperature Metalloids - also called semiconductors A. border the zigzag line B. about half of a complete s ...
... C. most are solid at room temperature Nonmetals A. found to the right of the zigzag line B. most have an almost complete set of electrons in the outer level C. More than half are gases at room temperature Metalloids - also called semiconductors A. border the zigzag line B. about half of a complete s ...
Periodic Table Notes Fill In
... 14. Where are the Transition Metals located? ______________________________________ 15. Where are the Lanthanides located? __________________________________________ 16. Where are the Actinides located? _____________________________________________ 17. Where are the Halogens located? _______________ ...
... 14. Where are the Transition Metals located? ______________________________________ 15. Where are the Lanthanides located? __________________________________________ 16. Where are the Actinides located? _____________________________________________ 17. Where are the Halogens located? _______________ ...
UNIT 3 –TEST REVIEW 1 Atoms of which of the
... Which of these groups (columns) in the Periodic Table is made up PRIMARILY of elements that are gases at room temperature? A Group 1 B Group 2 C Group 12 D Group 18 ...
... Which of these groups (columns) in the Periodic Table is made up PRIMARILY of elements that are gases at room temperature? A Group 1 B Group 2 C Group 12 D Group 18 ...
Groups of the Periodic Table
... 16. What is the difference between an electrical conductor and a thermal conductor? ...
... 16. What is the difference between an electrical conductor and a thermal conductor? ...
File
... c. The elements at the far left of the periodic table are nonmetals d. Elements are arranged by increasing atomic number 16. _____ Which of the following statements about alkali metals is true? a. Alkali metals are generally found in their uncombined form b. Alkali metals are group 1 elements c. Alk ...
... c. The elements at the far left of the periodic table are nonmetals d. Elements are arranged by increasing atomic number 16. _____ Which of the following statements about alkali metals is true? a. Alkali metals are generally found in their uncombined form b. Alkali metals are group 1 elements c. Alk ...
Grouping of Elements in the Periodic Table
... 7. Which elements are most likely to lose electrons and form cations? a) transition metals b) noble gases c) elements in the last two periods d) metals in the first two periods 8. What is another name for semimetals? a) alkaline earth metals b) alkali metals c) transition metals d) metalloids 9. How ...
... 7. Which elements are most likely to lose electrons and form cations? a) transition metals b) noble gases c) elements in the last two periods d) metals in the first two periods 8. What is another name for semimetals? a) alkaline earth metals b) alkali metals c) transition metals d) metalloids 9. How ...
Chemistry
... Compound – when two or more elements combine chemically and form a new substance. Compounds have three important characteristics (properties): (1) Compounds have a definite composition (2) Compounds can be broken down into simpler substances by chemical means and (3) Compounds can be identified by t ...
... Compound – when two or more elements combine chemically and form a new substance. Compounds have three important characteristics (properties): (1) Compounds have a definite composition (2) Compounds can be broken down into simpler substances by chemical means and (3) Compounds can be identified by t ...
Unit 10: Chemical Periodicity
... inner transition metal ionization energy noble gas period periodic law representative element transition metal Know s, p, d, and f block and what other names these areas are called (transition, inner transition, representative elements, etc) (also alkali, alkaline earth, halogen, noble gas locatio ...
... inner transition metal ionization energy noble gas period periodic law representative element transition metal Know s, p, d, and f block and what other names these areas are called (transition, inner transition, representative elements, etc) (also alkali, alkaline earth, halogen, noble gas locatio ...
HonorsCh6PracticeTest14
... Choose the best answer and write its letter on the line. _______ 11. In the modern periodic table, there is a periodic pattern in the physical and chemical properties of elements when they are arranged in order of a. increasing atomic mass. b. increasing electronegativity. c. increasing atomic radiu ...
... Choose the best answer and write its letter on the line. _______ 11. In the modern periodic table, there is a periodic pattern in the physical and chemical properties of elements when they are arranged in order of a. increasing atomic mass. b. increasing electronegativity. c. increasing atomic radiu ...
The Atom and how it is organized - Cashmere
... The atoms of all elements are made up of a central nucleus with orbiting electrons. ◦ A nucleus is made up of positively charged PROTONS and neutral NEUTRONS. ◦ ELECTRONS are negatively charged and orbit around the nucleus. ...
... The atoms of all elements are made up of a central nucleus with orbiting electrons. ◦ A nucleus is made up of positively charged PROTONS and neutral NEUTRONS. ◦ ELECTRONS are negatively charged and orbit around the nucleus. ...
6-Getting to Know the Periodic Table
... 3) Using black ink OR pencil, write in the period number for each period, and group number for each group. 4) Answer each of the following questions: a. Which element has 27 protons in its nucleus? ___________________________ b. How many neutrons does Phosphorous have? _________ c. What is the famil ...
... 3) Using black ink OR pencil, write in the period number for each period, and group number for each group. 4) Answer each of the following questions: a. Which element has 27 protons in its nucleus? ___________________________ b. How many neutrons does Phosphorous have? _________ c. What is the famil ...
alkaline earth metals
... Groups of Metals • Alkali metals and alkaline earth metals –at left of the PT and very reactive • Transition metals—near the center of the PT and include copper, gold, silver, and iron • Rare earth metals– in the top row of the 2 rows of metals show outside the main body of the PT • Two bottom rows ...
... Groups of Metals • Alkali metals and alkaline earth metals –at left of the PT and very reactive • Transition metals—near the center of the PT and include copper, gold, silver, and iron • Rare earth metals– in the top row of the 2 rows of metals show outside the main body of the PT • Two bottom rows ...
Physical properties
... substance is not shiny a physical property of metals that allows them to be hammered into different shapes a physical property of metals that allows them to be drawn out into a wire a physical property that allows substances to break or shatter easily a physical property of substances that allows he ...
... substance is not shiny a physical property of metals that allows them to be hammered into different shapes a physical property of metals that allows them to be drawn out into a wire a physical property that allows substances to break or shatter easily a physical property of substances that allows he ...
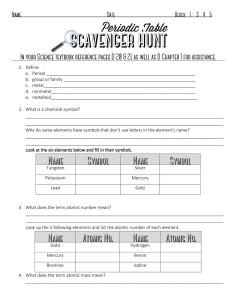
1. Define: a. Period b. group or family
... 10. What are most elements: metals, non-metals or metalloids?_______________________________ 11. Complete the chart below: ...
... 10. What are most elements: metals, non-metals or metalloids?_______________________________ 11. Complete the chart below: ...
How is an Atoms Structure Related to its Position on the Periodic
... How is an Atoms Structure Related to its Position on the Periodic Table? http://goo.gl/Cf5SvM ...
... How is an Atoms Structure Related to its Position on the Periodic Table? http://goo.gl/Cf5SvM ...
Mr. Trachtenberg`s Big Chemistry Test Review Part I of III 20pts
... 11.) Elements in the upper right-‐hand corner of the Periodic Table have the highest electronegativies and ionization energies. 12.) Moving down a group atoms get larger 13.) Moving from l ...
... 11.) Elements in the upper right-‐hand corner of the Periodic Table have the highest electronegativies and ionization energies. 12.) Moving down a group atoms get larger 13.) Moving from l ...