nuclear chemistry
... o 92238U 90234 Th + 42 He TYPES OF RADIOACTIVE DECAY 3 Types o Alpha radiation is the loss of a He particle o Beta radiation is the loss of an electron o Gamma radiation is the loss of a photon Nucleons can undergo 2 other types of decay o Positron emission ( same mass as an electron but positi ...
... o 92238U 90234 Th + 42 He TYPES OF RADIOACTIVE DECAY 3 Types o Alpha radiation is the loss of a He particle o Beta radiation is the loss of an electron o Gamma radiation is the loss of a photon Nucleons can undergo 2 other types of decay o Positron emission ( same mass as an electron but positi ...
Photo chapter opener 21 Subatomic particle tracks in a bubble
... • A nuclide is a type of atom characterized by its proton number, neutron number and its energy condition. • Nuclides with identical proton number but differing neutron number are called isotopes. • Conditions with a life of less than 10-10s are called excited conditions of a nuclide. • At present, ...
... • A nuclide is a type of atom characterized by its proton number, neutron number and its energy condition. • Nuclides with identical proton number but differing neutron number are called isotopes. • Conditions with a life of less than 10-10s are called excited conditions of a nuclide. • At present, ...
Principles of Matter and Energy
... By determining ratios of the different isotopes of carbon and other elements in samples of biological origin and in rocks, scientists are able to determine with certainty when these materials formed. ...
... By determining ratios of the different isotopes of carbon and other elements in samples of biological origin and in rocks, scientists are able to determine with certainty when these materials formed. ...
Serway_PSE_quick_ch45
... reactions. Once the hydrogen has been exhausted, fusion of helium nuclei can occur. Once the helium is used up, if the star is sufficiently massive, fusion of heavier and heavier nuclei can occur. Consider fusion reactions involving two nuclei with the same value of A. For these types of reactions, ...
... reactions. Once the hydrogen has been exhausted, fusion of helium nuclei can occur. Once the helium is used up, if the star is sufficiently massive, fusion of heavier and heavier nuclei can occur. Consider fusion reactions involving two nuclei with the same value of A. For these types of reactions, ...
I Examen II trim Science
... 9. Explain the three types of radioactive decay. The nucleus of all atoms (with the exception of hydrogen) contains one or more protons and one or more neutrons. The nucleus of most carbon atoms, for instance, contains six protons and six neutrons. In most cases, the nuclei of atoms are stable; that ...
... 9. Explain the three types of radioactive decay. The nucleus of all atoms (with the exception of hydrogen) contains one or more protons and one or more neutrons. The nucleus of most carbon atoms, for instance, contains six protons and six neutrons. In most cases, the nuclei of atoms are stable; that ...
Fission and Fusion
... • Fission is the breaking up of an unstable uranium atom. • Fission is easier to start and control than fusion, but produces less energy and generates highly radioactive waste. • In uncontrolled fission nuclear chain reactions occur resulting in a large explosion. • In controlled fission water or gr ...
... • Fission is the breaking up of an unstable uranium atom. • Fission is easier to start and control than fusion, but produces less energy and generates highly radioactive waste. • In uncontrolled fission nuclear chain reactions occur resulting in a large explosion. • In controlled fission water or gr ...
Nuc Chem PP - Liberty Union High School District
... A sample of 3x107 Radon atoms are trapped in a basement that is sealed. The half-life of Radon is 3.83 days. How many radon atoms are left after 31 days? answer:1.2x105 atoms ...
... A sample of 3x107 Radon atoms are trapped in a basement that is sealed. The half-life of Radon is 3.83 days. How many radon atoms are left after 31 days? answer:1.2x105 atoms ...
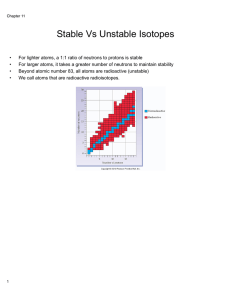
Stable Vs Unstable Isotopes
... Which of the properties of radioisotopes make them useful as tracers in medical or agricultural applications? I. Their chemical behavior is the same as nonradioactive isotopes. II. They emit various types of radiation. III. The nuclear reaction is unaffected by the chemical state of the isotope. ...
... Which of the properties of radioisotopes make them useful as tracers in medical or agricultural applications? I. Their chemical behavior is the same as nonradioactive isotopes. II. They emit various types of radiation. III. The nuclear reaction is unaffected by the chemical state of the isotope. ...
Radioactivity - Williamstown Independent Schools
... materials that can be safely placed in the body to track movement of materials. • Radiation can also be used to kill cancerous ...
... materials that can be safely placed in the body to track movement of materials. • Radiation can also be used to kill cancerous ...
Radioactive Decay Laws
... Now whenever mass disappears energy is created, according to Einstein's formula E = mc2, and... the mass was just equivalent to 200 MeV; it all fitted! Meitner was convinced that the product actually was Barium rather than a homologue. The nightmare of contradictory evidence all fit the explanation ...
... Now whenever mass disappears energy is created, according to Einstein's formula E = mc2, and... the mass was just equivalent to 200 MeV; it all fitted! Meitner was convinced that the product actually was Barium rather than a homologue. The nightmare of contradictory evidence all fit the explanation ...
Document
... 1. The amount of material left after two half-lives is _one-fourth (1/4) _ of the original amount. 2. _Fission_ means "to divide." 3. _Nuclear Fusion_ is the combining of two low-mass nuclei into one nucleus with a larger mass. 4. Radioactive isotopes that are put into the body to monitor a bodily p ...
... 1. The amount of material left after two half-lives is _one-fourth (1/4) _ of the original amount. 2. _Fission_ means "to divide." 3. _Nuclear Fusion_ is the combining of two low-mass nuclei into one nucleus with a larger mass. 4. Radioactive isotopes that are put into the body to monitor a bodily p ...
nuclear chemistry - Wood County Schools
... Fission: The splitting of an atomic nucleus into two or more smaller fragments. Fusion: The combining of two atoms to form an element of a larger atomic number. Releases more energy than fission. Chain Reaction: A self-sustaining fission process. Critical Mass: The minimum quantity of a radioactive ...
... Fission: The splitting of an atomic nucleus into two or more smaller fragments. Fusion: The combining of two atoms to form an element of a larger atomic number. Releases more energy than fission. Chain Reaction: A self-sustaining fission process. Critical Mass: The minimum quantity of a radioactive ...
Nuclear Chemistry
... Now we add up mass numbers and atomic numbers. (201 + 0 = 201 and 80 + -1 =79). Element 79 is gold, so the answer is: 20180Hg + 0-1e -->20179Au ...
... Now we add up mass numbers and atomic numbers. (201 + 0 = 201 and 80 + -1 =79). Element 79 is gold, so the answer is: 20180Hg + 0-1e -->20179Au ...
physics 30 Matter assignment 4 - ND
... 14. The half-life of a theoretical radioactive isotope is 11.6 h. If there are 5.5 x 1022 atoms initially, how many atoms will remain after 48 h? a. ...
... 14. The half-life of a theoretical radioactive isotope is 11.6 h. If there are 5.5 x 1022 atoms initially, how many atoms will remain after 48 h? a. ...
Unit IV Review Guide: Atomic Structure and Nuclear Reactions
... 10. What change in mass number occurs when a radioactive atom emits an alpha particle? A beta particle? A gamma particle? ...
... 10. What change in mass number occurs when a radioactive atom emits an alpha particle? A beta particle? A gamma particle? ...
Nuclear Fission and Fusion
... Nuclear Fission and Energy: The splitting of atoms produces a large amount of energy. This energy is produced in nuclear power plants Problems with nuclear power: The waste produced has to be safely stored for thousands of years. ...
... Nuclear Fission and Energy: The splitting of atoms produces a large amount of energy. This energy is produced in nuclear power plants Problems with nuclear power: The waste produced has to be safely stored for thousands of years. ...
What do I know about……
... understand that a nucleus of U-235 can be split (the process of fission) by collision with a neutron, and that this process releases energy in the form of kinetic energy of the fission products recall that the fission of U-235 produces two daughter nuclei and a small number of neutrons understand th ...
... understand that a nucleus of U-235 can be split (the process of fission) by collision with a neutron, and that this process releases energy in the form of kinetic energy of the fission products recall that the fission of U-235 produces two daughter nuclei and a small number of neutrons understand th ...
Pre-Knowledge: Chemistry and Physics Vocabulary Atomic Number
... isotope is predictable. That is, we cannot predict when a specific atom will decay, but after one half-life half of the atoms will have decayed into a daughter nuclide. ...
... isotope is predictable. That is, we cannot predict when a specific atom will decay, but after one half-life half of the atoms will have decayed into a daughter nuclide. ...
NUCLEAR CHEMISTRY REVIEW SHEET
... _____15. Half-life is the length of time in which half of the atoms in a sample a. undergo radioactive decay b. undergo nuclear fission c. undergo nuclear fusion d. react chemically _____ 16. When an atom releases energy through gamma rays, what properties of the nucleus are changed? a. both mass nu ...
... _____15. Half-life is the length of time in which half of the atoms in a sample a. undergo radioactive decay b. undergo nuclear fission c. undergo nuclear fusion d. react chemically _____ 16. When an atom releases energy through gamma rays, what properties of the nucleus are changed? a. both mass nu ...
PreAP Chemistry Radioactivity WS Name Period ____ Match the
... ---------- 5. The weighted average mass of an element’s isotopes. ...
... ---------- 5. The weighted average mass of an element’s isotopes. ...
Nuclear Chemistry - VCC Library
... neutrons as a group. Nuclide is similar to isotope (atoms of the same element with different masses) but broader in definition—it includes ANY isotope of ANY atom. PARTICLES INVOLVED IN NUCLEAR REACTIONS The three principal emissions from the nuclei of radioactive elements are alpha particles, beta ...
... neutrons as a group. Nuclide is similar to isotope (atoms of the same element with different masses) but broader in definition—it includes ANY isotope of ANY atom. PARTICLES INVOLVED IN NUCLEAR REACTIONS The three principal emissions from the nuclei of radioactive elements are alpha particles, beta ...
Nuclear Chemistry - Mona Shores Blogs
... • Because of the size issue, there is a point beyond which all elements are radioactive. • Once they become large enough, the ...
... • Because of the size issue, there is a point beyond which all elements are radioactive. • Once they become large enough, the ...