* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Fission and Fusion
Valley of stability wikipedia , lookup
Atomic nucleus wikipedia , lookup
Fusion power wikipedia , lookup
Nuclear binding energy wikipedia , lookup
Inertial electrostatic confinement wikipedia , lookup
Muon-catalyzed fusion wikipedia , lookup
Inertial confinement fusion wikipedia , lookup
Nuclear fusion wikipedia , lookup
Nuclear fission wikipedia , lookup
Nuclear transmutation wikipedia , lookup
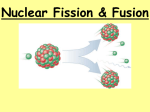
Fission and Fusion They could be described as “Big Bang” and “Bigger Bang”. Learning Objectives • Compare the nuclear changes, fuel used and products formed in fission and fusion reactions. • Identify common examples of fission and fusion reactions. • Define critical mass. • Describe the difference in and net results of controlled and uncontrolled fission reactions. • Use Einstein’s E-mc2 equation to relate the mass lost to the energy production in nuclear reactions. Fission and Fusion Reactions Nuclear Fission Nuclear Fusion • Large unstable atom splits apart • Small atoms fuse together • Requires minimum (critical) mass • Requires 100,000,000° temperature • Produces a variety of toxic products • Produces Helium as product • Fuel-Uranium-235 or Plutonium-244 • Fuel-Hydrogen-2 or Hydrogen-3 • Used in power plants and ships • Reaction in sun and other stars • Produces more energy per pound Controlled and Uncontrolled Fission • In the fission reaction, when the fuel atom splits, several neutrons are released. • If enough fuel atoms (critical mass) are present the neutrons strike other fuel atoms causing them to become unstable and split, continuing the process. • Uncontrolled - If the reaction is allowed to continue it speeds up out of control……Big Boom! • Controlled - In nuclear reactors insulating materials (control rods) are used to absorb most of the neutrons, allowing the reaction to proceed slowly. The result is the production of useable amounts of heat energy. E = mc2 • In both fission and fusion reactions a small amount of mass in the nucleus is changed to energy. • Einstein’s equation can be used to predict the amount of energy produced by fission and fusion processes. • Because the term c2 is so large (9x1016) a very very small amount of mass is converted into a very very large amount of energy. • 1 gram of mass lost = 90,000,000,000,000 joules of energy Nuclear Power Plants Worldwide Fission Reaction Fusion Reaction Key Concepts Review • Nuclear reactions produce tremendous amounts of usable heat energy. • Fission is the breaking up of an unstable uranium atom. • Fission is easier to start and control than fusion, but produces less energy and generates highly radioactive waste. • In uncontrolled fission nuclear chain reactions occur resulting in a large explosion. • In controlled fission water or graphite are used to absorb some of the neutrons produced by fission, controlling the rate of the fission process. • Fusion is the fusing of two unstable hydrogen atoms to form helium. • Fusion is harder to start and control than fission, but produces more energy per pound of fuel and produces no toxic waste products. • There are 120+ nuclear power plants worldwide producing 20% of the world’s electricity