Chapter 7 - Bakersfield College
... D. Binding energy makes stable heavier nuclei possible (beyond hydrogen) which in turn accounts for the various elements and forms of matter found in the physical universe. 7-9. Nuclear Fission A. In 1939, uranium-235 was discovered to undergo nuclear fission when struck by a neutron. 1. A nucleus o ...
... D. Binding energy makes stable heavier nuclei possible (beyond hydrogen) which in turn accounts for the various elements and forms of matter found in the physical universe. 7-9. Nuclear Fission A. In 1939, uranium-235 was discovered to undergo nuclear fission when struck by a neutron. 1. A nucleus o ...
Outline Chapter 8 The Nucleus 8-1. J.J. Thompson`s Plum Pudding
... The binding energy per nucleon is found by dividing the total binding energy of the nucleus by the number of nucleons (protons and neutrons) it contains; the greater the binding energy per nucleon, the more stable the nucleus. ...
... The binding energy per nucleon is found by dividing the total binding energy of the nucleus by the number of nucleons (protons and neutrons) it contains; the greater the binding energy per nucleon, the more stable the nucleus. ...
HW-1-Ch1-Atomic-structure-W16
... 35. Penetration & Shielding of an Electron in multi-electron atom and how does it affect the filling order as given by “Building Up” principle? ...
... 35. Penetration & Shielding of an Electron in multi-electron atom and how does it affect the filling order as given by “Building Up” principle? ...
Radioactivity - Science 9
... The nucleus contains a huge amount of energy that holds it all together. When this energy is released, a large amount can be gathered from the tiny mass. NUCLEAR FISSION: The splitting of an atomic nucleus into two smaller nuclei of approximately equal mass. This does not happen spontaneously, b ...
... The nucleus contains a huge amount of energy that holds it all together. When this energy is released, a large amount can be gathered from the tiny mass. NUCLEAR FISSION: The splitting of an atomic nucleus into two smaller nuclei of approximately equal mass. This does not happen spontaneously, b ...
nuclear reactions
... In nuclear reactions, the total numbers of nucleons – protons, neutrons and electrons – remains constant, but chemical identity can change when the particles interchange Nuclear reactions permit the transmutation of elements Balancing Nuclear Reactions (1) The sum of the mass numbers of reacting nuc ...
... In nuclear reactions, the total numbers of nucleons – protons, neutrons and electrons – remains constant, but chemical identity can change when the particles interchange Nuclear reactions permit the transmutation of elements Balancing Nuclear Reactions (1) The sum of the mass numbers of reacting nuc ...
NUCLEAR CHEMISTRY
... normally strong enough to hold the protons and neutrons together. However, sometimes the force of repulsion due to the protons having the same charge overcomes the strong nuclear force and the atom breaks apart. ...
... normally strong enough to hold the protons and neutrons together. However, sometimes the force of repulsion due to the protons having the same charge overcomes the strong nuclear force and the atom breaks apart. ...
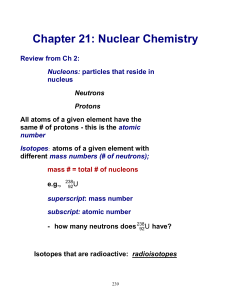
Chapter 21: Nuclear Chemistry
... We plot # of neutrons v. number of protons: n/p ratio where stable nuclei reside is called the 'belt of stability' belt of stability ends at atomic number 83: all nuclei with atomic number 84 are radioactive ...
... We plot # of neutrons v. number of protons: n/p ratio where stable nuclei reside is called the 'belt of stability' belt of stability ends at atomic number 83: all nuclei with atomic number 84 are radioactive ...
Review 1st Qtr KEY
... PART I: You should know the basics (terms & basic facts) about… Hypothesis theory law scientific method mass weight volume physical change chemical change mixture element compound molecule heterogeneous homogeneous states of matter ...
... PART I: You should know the basics (terms & basic facts) about… Hypothesis theory law scientific method mass weight volume physical change chemical change mixture element compound molecule heterogeneous homogeneous states of matter ...
Glossary of Technical Terms - Institute for Energy and Environmental
... A material consisting of atoms whose nuclei can be split when irradiated with low energy (ideally, zero energy) neutrons. Well-known examples are plutonium-239 and uranium-235. fast reactor A reactor that is designed to use fast neutrons for sustaining the nuclear chain reaction. Fast reactors can b ...
... A material consisting of atoms whose nuclei can be split when irradiated with low energy (ideally, zero energy) neutrons. Well-known examples are plutonium-239 and uranium-235. fast reactor A reactor that is designed to use fast neutrons for sustaining the nuclear chain reaction. Fast reactors can b ...
synthetic elements
... The rows of the table are known as periods. The columns of the table are known as groups. The table is divided into different bocks known as s-block ,pblock ,d-block ,f-block. The table can be used to derive relationships between the properties of the elements and predict the properties of n ...
... The rows of the table are known as periods. The columns of the table are known as groups. The table is divided into different bocks known as s-block ,pblock ,d-block ,f-block. The table can be used to derive relationships between the properties of the elements and predict the properties of n ...
RADIOACTIVITY involves the emission of energy and particles from
... The resultant changes might lead to a genetic mutation, to cancer, or to ...
... The resultant changes might lead to a genetic mutation, to cancer, or to ...
Unit 3 – Atomic Structure
... • Mass number -The total number of protons and neutrons in a nucleus • Subatomic particles -The three kinds of particles that make up atoms: protons, neutrons, and electrons. • Nuclear fission - Splitting of the nucleus into smaller nuclei • Nuclear fusion - Combining nuclei of light elements into a ...
... • Mass number -The total number of protons and neutrons in a nucleus • Subatomic particles -The three kinds of particles that make up atoms: protons, neutrons, and electrons. • Nuclear fission - Splitting of the nucleus into smaller nuclei • Nuclear fusion - Combining nuclei of light elements into a ...
A Conceptual Introduction to Chemistry, First Edition
... neutrons will not escape due to energy that is higher than optimum for inducing further fission A chain reaction should maintain a constant rate Critical mass – The smallest amount of fissionable material necessary to support a continuing chain reaction ...
... neutrons will not escape due to energy that is higher than optimum for inducing further fission A chain reaction should maintain a constant rate Critical mass – The smallest amount of fissionable material necessary to support a continuing chain reaction ...
Isotopes
... a mix of those four types (details here) The early suns also made radioactive isotopes of iron and pretty much anything else. But those non-stable guys have long since decayed, except if their half-life is billion of years. That's why we still have, for example, some uranium (U) around. 238U, to giv ...
... a mix of those four types (details here) The early suns also made radioactive isotopes of iron and pretty much anything else. But those non-stable guys have long since decayed, except if their half-life is billion of years. That's why we still have, for example, some uranium (U) around. 238U, to giv ...
Document
... - the probability that one nucleus will decay in a unit time is defined as (units of s-1, y-1) - If we have N unstable nuclei, the number of decays in time dt is ...
... - the probability that one nucleus will decay in a unit time is defined as (units of s-1, y-1) - If we have N unstable nuclei, the number of decays in time dt is ...
Nuclear Radiation1516
... NUCLEAR FISSION When a nucleus fissions, it splits into several smaller fragments. These fragments, or fission products, are about equal to half the original mass. Two or three neutrons are also emitted. The sum of the masses of these fragments is less than the original mass. This 'missing' mass (a ...
... NUCLEAR FISSION When a nucleus fissions, it splits into several smaller fragments. These fragments, or fission products, are about equal to half the original mass. Two or three neutrons are also emitted. The sum of the masses of these fragments is less than the original mass. This 'missing' mass (a ...
Review and Radioactivity
... A nucleus which is in an excited state may emit one or more photons (packets of electromagnetic radiation) of discrete energies. The emission of gamma rays does not alter the number of protons or neutrons in the nucleus but instead has the effect of moving the nucleus from a higher to a lower energy ...
... A nucleus which is in an excited state may emit one or more photons (packets of electromagnetic radiation) of discrete energies. The emission of gamma rays does not alter the number of protons or neutrons in the nucleus but instead has the effect of moving the nucleus from a higher to a lower energy ...
Nuclear - PEO Scarborough Chapter
... neutrons. The electrons form a cloud around the nucleus. The neutrons hold mass containing particles (protons) together in the nucleus. When an atom has too much energy, it dissipates energy through emission of alpha (α), beta (β) and gamma (γ) radiation. This phenomenon is called radioactivity or r ...
... neutrons. The electrons form a cloud around the nucleus. The neutrons hold mass containing particles (protons) together in the nucleus. When an atom has too much energy, it dissipates energy through emission of alpha (α), beta (β) and gamma (γ) radiation. This phenomenon is called radioactivity or r ...
Santee Education Complex Chemistry Mini Assessment 11
... b. 0n1 + 13Al27 → 11Na24 + 2He4 c. 13Al27 + 2He4 → 15P30 +0n1 d. 7N14 + 2He4 →1H1 + 8O17 14) A process in which a very heavy nucleus splits into more stable nuclei of intermediate mass is called: a. nuclear fission. b. a chain reaction. c. nuclear fusion. d. radiocarbon dating. 15) An electron emitt ...
... b. 0n1 + 13Al27 → 11Na24 + 2He4 c. 13Al27 + 2He4 → 15P30 +0n1 d. 7N14 + 2He4 →1H1 + 8O17 14) A process in which a very heavy nucleus splits into more stable nuclei of intermediate mass is called: a. nuclear fission. b. a chain reaction. c. nuclear fusion. d. radiocarbon dating. 15) An electron emitt ...
Nuclear power LEDE-HISTORY version C 1. Fermi thought he had
... 21. The worldwide number of nuclear reactors and their net capacity grew steadily from 1960, and a) did not begin to level off until Chernobyl (1986) b) leveled off between Three Mile Island (1979) and Chernobyl (1986). c) fluctuated randomly but with a strong correlation with the world economy and ...
... 21. The worldwide number of nuclear reactors and their net capacity grew steadily from 1960, and a) did not begin to level off until Chernobyl (1986) b) leveled off between Three Mile Island (1979) and Chernobyl (1986). c) fluctuated randomly but with a strong correlation with the world economy and ...
Scientists` Consensus Ideas Atomic Structure and Nuclear Interactions
... quantities of energy compared to chemical reactions. 14. Some elements change into other elements as a result of nuclear reactions. Physical and chemical interactions do not convert one element into another element. 15. Nuclear radiation refers to the particles and energy released during nuclear rea ...
... quantities of energy compared to chemical reactions. 14. Some elements change into other elements as a result of nuclear reactions. Physical and chemical interactions do not convert one element into another element. 15. Nuclear radiation refers to the particles and energy released during nuclear rea ...
Atomic and Nuclear Physics
... with a neutron to produce 2 daughter nuclei and a small number of neutrons (3) • This process releases energy in the form of kinetic energy (= thermal energy) of the 2 nuclei (fission products) • The neutrons produced by one fission can strike other U-235 nuclei creating a chain reaction ...
... with a neutron to produce 2 daughter nuclei and a small number of neutrons (3) • This process releases energy in the form of kinetic energy (= thermal energy) of the 2 nuclei (fission products) • The neutrons produced by one fission can strike other U-235 nuclei creating a chain reaction ...
Powerpoint Slides
... • Objectives – Understand the concepts of ½ life and ½ thickness in radiation – Differentiate between fusion and fission – Describe the processes involved in radioactive decays (alpha, beta, and gamma) ...
... • Objectives – Understand the concepts of ½ life and ½ thickness in radiation – Differentiate between fusion and fission – Describe the processes involved in radioactive decays (alpha, beta, and gamma) ...
Chapter 25 Radioactivity
... Some nuclei are stable, some are unstable Larger nucleus = more unstable Smaller nucleus = more stable The nucleus of an atom is like a marble in the center of a football arena. The atom is composed of mostly space ...
... Some nuclei are stable, some are unstable Larger nucleus = more unstable Smaller nucleus = more stable The nucleus of an atom is like a marble in the center of a football arena. The atom is composed of mostly space ...