Word - chemmybear.com
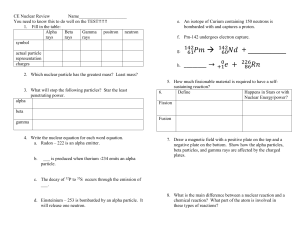
... 2. Pierre and Marie Curie invented a device to detect radiation because the radioactive material caused the air to _____________ electricity… thereby completing the circuit in the machine. 3. Radioactivity interacts with atoms in the air to form positive ions and free _______________. This makes the ...
... 2. Pierre and Marie Curie invented a device to detect radiation because the radioactive material caused the air to _____________ electricity… thereby completing the circuit in the machine. 3. Radioactivity interacts with atoms in the air to form positive ions and free _______________. This makes the ...
Atomic/Nuclear
... When a gamma ray is released, the same number of neutrons and protons are present but they have settled to a lower energy level. Alpha particles are stopped by air molecules in a few centimeters. Beta particles are stopped by air molecules in a few meters. Gamma rays can travel very long distances t ...
... When a gamma ray is released, the same number of neutrons and protons are present but they have settled to a lower energy level. Alpha particles are stopped by air molecules in a few centimeters. Beta particles are stopped by air molecules in a few meters. Gamma rays can travel very long distances t ...
Unit 7 Review
... Use the graph to explain why energy can be released in both the fission and the fusion processes ...
... Use the graph to explain why energy can be released in both the fission and the fusion processes ...
L37 - University of Iowa Physics
... holds the atom together • the neutrons and protons have about the same mass, and are each about 2000 times more massive than the electrons • the nucleus accounts for about 99.9% of the total mass of the atom • the neutrons have no charge what role ...
... holds the atom together • the neutrons and protons have about the same mass, and are each about 2000 times more massive than the electrons • the nucleus accounts for about 99.9% of the total mass of the atom • the neutrons have no charge what role ...
File
... A neutral atom consists of a positively charged nucleus (composed of protons and neutrons) associated with orbital electrons. The atomic number (Z) is the number of protons in the nucleus The neutron number (N) is the number of neutrons in the nucleus. The mass number (A) is the sum of the protons a ...
... A neutral atom consists of a positively charged nucleus (composed of protons and neutrons) associated with orbital electrons. The atomic number (Z) is the number of protons in the nucleus The neutron number (N) is the number of neutrons in the nucleus. The mass number (A) is the sum of the protons a ...
ib atomic and nuclear physics definitions and concepts
... NUCLEON: The particles in the nucleus (proton or neutron). NUCLEON NUMBER, A: The number of nucleons in the nucleus. PROTON NUMBER, Z: The number of protons in the nucleus. NEUTRON NUMBER, N: duh! N=A-Z RADIOACTIVE DECAY: A random and spontaneous process in which unstable nuclei emit a particle (dis ...
... NUCLEON: The particles in the nucleus (proton or neutron). NUCLEON NUMBER, A: The number of nucleons in the nucleus. PROTON NUMBER, Z: The number of protons in the nucleus. NEUTRON NUMBER, N: duh! N=A-Z RADIOACTIVE DECAY: A random and spontaneous process in which unstable nuclei emit a particle (dis ...
Lanthanoids and actinoids
... Because the three stages of ionization enthalpy of lanthanoid elements are comparatively low, they are positive elements and readily assume trivalent ionic states. Most compounds of lanthanoids other than Ce4+ (4f0), Eu2+ (4f7), Yb2+ (4f14), are usually Ln3+ ones. Ln3+ species are hard acids, and si ...
... Because the three stages of ionization enthalpy of lanthanoid elements are comparatively low, they are positive elements and readily assume trivalent ionic states. Most compounds of lanthanoids other than Ce4+ (4f0), Eu2+ (4f7), Yb2+ (4f14), are usually Ln3+ ones. Ln3+ species are hard acids, and si ...
+ → ep no - University of Iowa Physics
... fusing deuterium with tritium nuclei to form helium and neutrons • To achieve this, the hydrogen must be heated to 100 million C using a fission bomb thermonuclear • Thermonuclear fusion is the energy source in a star ...
... fusing deuterium with tritium nuclei to form helium and neutrons • To achieve this, the hydrogen must be heated to 100 million C using a fission bomb thermonuclear • Thermonuclear fusion is the energy source in a star ...
2.10 Basic Nuclear Chemistry
... 2. Even numbers tend to be more stable than odd numbers of nucleons. B. As the number of protons in a nucleus increases, the stability of the nucleus decreases 1. This is because the positive repulsive forces are greater than the Nuclear Force. 2. To reduce this instability, neutrons are needed to i ...
... 2. Even numbers tend to be more stable than odd numbers of nucleons. B. As the number of protons in a nucleus increases, the stability of the nucleus decreases 1. This is because the positive repulsive forces are greater than the Nuclear Force. 2. To reduce this instability, neutrons are needed to i ...
21J 2011 The Polywell Nuclear Reactor Website July 4, 2011
... atom, and giving the rest of the atom a net positive electrical charge. Neutrons can cause already existing chemicals in air, water or other nearby materials to become unstable and radioactive. As these unstable forms of natural materials return to their normal stable state, they also can release io ...
... atom, and giving the rest of the atom a net positive electrical charge. Neutrons can cause already existing chemicals in air, water or other nearby materials to become unstable and radioactive. As these unstable forms of natural materials return to their normal stable state, they also can release io ...
Document
... Radionuclides are nuclei that are radioactive – i.e., they will spontaneously emit radiation. Atoms containing these nuclei are called radioisotopes. ...
... Radionuclides are nuclei that are radioactive – i.e., they will spontaneously emit radiation. Atoms containing these nuclei are called radioisotopes. ...
Chemistry 102B What`s in an atom? Before “Chemistry” Other Early
... Before “Chemistry” • Alchemy/Alchemists - a pseudoscience built around trying to turn cheap metals into GOLD! (400 B.C.-1400 A.D.) • Metallurgy – systematic extraction of metals from ores laid some groundwork for modern chemistry. (1500s) • The first “chemist” was Robert Boyle who worked on pressure ...
... Before “Chemistry” • Alchemy/Alchemists - a pseudoscience built around trying to turn cheap metals into GOLD! (400 B.C.-1400 A.D.) • Metallurgy – systematic extraction of metals from ores laid some groundwork for modern chemistry. (1500s) • The first “chemist” was Robert Boyle who worked on pressure ...
Radioactivity and Nuclear Physics
... Alpha decay is the emission of a helium nucleus, causing the product to have an atomic number 2 less than the original and an atomic mass number 4 less than the original. Beta minus decay is the emission of an electron, causing the product to have an atomic number 1 greater than the original Beta pl ...
... Alpha decay is the emission of a helium nucleus, causing the product to have an atomic number 2 less than the original and an atomic mass number 4 less than the original. Beta minus decay is the emission of an electron, causing the product to have an atomic number 1 greater than the original Beta pl ...
Exam II
... Radioactive nuclear waste should be permanently stored in rocks that are mixed with water to provide a radiation-absorbing barrier very dry to prevent corrosion and leaking of radiation. ...
... Radioactive nuclear waste should be permanently stored in rocks that are mixed with water to provide a radiation-absorbing barrier very dry to prevent corrosion and leaking of radiation. ...
Nuclear Processes
... When a radioactive nucleus such as U23892 decays it often produces another radioactive isotope which goes on to decay further. ...
... When a radioactive nucleus such as U23892 decays it often produces another radioactive isotope which goes on to decay further. ...
Unit 3 Study Guide: Atomic Structure and Nuclear
... In a nuclear power plant, energy is produced in the reactor core by fission reactions that occur inuraniumcontaining bars called (18) ____________________. The uranium is found at location (19)_________________ in the diagram. The rate at which the nuclear reaction takes place is controlled by other ...
... In a nuclear power plant, energy is produced in the reactor core by fission reactions that occur inuraniumcontaining bars called (18) ____________________. The uranium is found at location (19)_________________ in the diagram. The rate at which the nuclear reaction takes place is controlled by other ...
1 0 +1 0 - davis.k12.ut.us
... occur in nature, can you list a few? Technetium, neptunium, elements 93 and up… 10. How can synthetic elements be produced? Bombarding an atom with a neutron or alpha particle. 11. A 5.0 g sample of Lead-210 decays to approximately .63 g. How much time has passed if Lead-210 has a half-life of 22 ye ...
... occur in nature, can you list a few? Technetium, neptunium, elements 93 and up… 10. How can synthetic elements be produced? Bombarding an atom with a neutron or alpha particle. 11. A 5.0 g sample of Lead-210 decays to approximately .63 g. How much time has passed if Lead-210 has a half-life of 22 ye ...
Basics of Nuclear Physics and Fission
... Spontaneous fission, which is the fission of a heavy element without input of any external particle or energy. Often, there is still excess residual energy in the nucleus after the emission of a particle or after electron capture. Some of this residual energy after radioactive decay can be emitted i ...
... Spontaneous fission, which is the fission of a heavy element without input of any external particle or energy. Often, there is still excess residual energy in the nucleus after the emission of a particle or after electron capture. Some of this residual energy after radioactive decay can be emitted i ...
Periodic Table
... Metalloids • __________ have some chemical and physical properties of metals and other properties of nonmetals. ...
... Metalloids • __________ have some chemical and physical properties of metals and other properties of nonmetals. ...
Nuclear Chemistry
... The nucleus can ‘capture’ one of its own innerorbital electrons if the atom is unstable due to too many protons. The electron will combine with a proton in the nucleus and form a neutron. The atomic number decreases by one but the mass number stays the same. ...
... The nucleus can ‘capture’ one of its own innerorbital electrons if the atom is unstable due to too many protons. The electron will combine with a proton in the nucleus and form a neutron. The atomic number decreases by one but the mass number stays the same. ...
Nuclear Weapons (and Energy) Each element has different number
... Electron (negative charge) – charge = -1.6 x 10-19 Coulombs ...
... Electron (negative charge) – charge = -1.6 x 10-19 Coulombs ...
SMART Notebook
... - But, the little bit of carbon-13 does pull up the number a bit, so we get 12.01u. - The number of neutrons strongly affects the stability of the nucleus. This is why some isotopes are more common than others. - Because different isotopes have different masses, they can be separated from each other ...
... - But, the little bit of carbon-13 does pull up the number a bit, so we get 12.01u. - The number of neutrons strongly affects the stability of the nucleus. This is why some isotopes are more common than others. - Because different isotopes have different masses, they can be separated from each other ...
Nuclear chemistry – the study of nuclear reactions and their uses in
... ii. Greater amounts of energy are released if very light nuclei are combined or fused together to give more massive nuclei 1. This process is call fusion (exothermic process) and is the essential energy producing process in the Sun. ...
... ii. Greater amounts of energy are released if very light nuclei are combined or fused together to give more massive nuclei 1. This process is call fusion (exothermic process) and is the essential energy producing process in the Sun. ...