Fission and Fusion
... In this example, a stray neutron strikes an atom of U-235. It absorbs the neutron and becomes an unstable atom of U-236. It then undergoes fission. Notice that more neutrons are released in the reaction. These neutrons can strike other U-235 atoms to initiate their fission. ...
... In this example, a stray neutron strikes an atom of U-235. It absorbs the neutron and becomes an unstable atom of U-236. It then undergoes fission. Notice that more neutrons are released in the reaction. These neutrons can strike other U-235 atoms to initiate their fission. ...
Inside the Atom connections to the lower secondary (KS3
... • conservation of mass changes of state and chemical reactions. Most of the nuclear physics related content in the KS3 curriculum is taught in the chemistry modules. Students are introduced to a simple atomic model and chemical reactions as the rearrangements of atoms. Using this as a starting poin ...
... • conservation of mass changes of state and chemical reactions. Most of the nuclear physics related content in the KS3 curriculum is taught in the chemistry modules. Students are introduced to a simple atomic model and chemical reactions as the rearrangements of atoms. Using this as a starting poin ...
NOTES: 2.1 - Intro to Chemistry
... The Chemistry of Life… ● when you breathe, eat, or drink, your body uses the substances in air, food, and water to carry out chemical reactions that keep you alive… ● just as an architect first must understand the building materials, a BIOLOGIST must first understand the compounds that make up livi ...
... The Chemistry of Life… ● when you breathe, eat, or drink, your body uses the substances in air, food, and water to carry out chemical reactions that keep you alive… ● just as an architect first must understand the building materials, a BIOLOGIST must first understand the compounds that make up livi ...
ppt-nuclear - SandersScienceStuff
... • Nuclear power plants have to be very precise in their regulations of the reactions. Some of the products of the fission reaction are extremely radioactive. • To ensure safety of all living things, the waste must be properly stored. • It can take up to twenty half-lives for such radioactivity to re ...
... • Nuclear power plants have to be very precise in their regulations of the reactions. Some of the products of the fission reaction are extremely radioactive. • To ensure safety of all living things, the waste must be properly stored. • It can take up to twenty half-lives for such radioactivity to re ...
Student 5
... What conclusions were made about the atom from Rutherford’s gold leaf experiment? Rutherford fired a beam of alpha particles at thin gold foil. The alpha particles were from a radioactive source, in an evacuated container. A scintillation detector then rotated around the container was used to pick u ...
... What conclusions were made about the atom from Rutherford’s gold leaf experiment? Rutherford fired a beam of alpha particles at thin gold foil. The alpha particles were from a radioactive source, in an evacuated container. A scintillation detector then rotated around the container was used to pick u ...
Phys 282 EXP 8
... 4.28% mass 113. (Using the nuclear masses of 114.9041 and 112.9043 instead of the number of nucleons, 115 and 113, the chemical weight of 114.82 can be calculated.) If the indium is placed where there are many free neutrons with kinetic energies, on the order of 0.03 eV, both isotopes In-115 and In- ...
... 4.28% mass 113. (Using the nuclear masses of 114.9041 and 112.9043 instead of the number of nucleons, 115 and 113, the chemical weight of 114.82 can be calculated.) If the indium is placed where there are many free neutrons with kinetic energies, on the order of 0.03 eV, both isotopes In-115 and In- ...
Ch 21 Nuclear - coolchemistrystuff
... Radioactive series (or nuclear disintegration series): a series of nuclear reactions that begins with an unstable nucleus and terminates with a stable one Magic numbers: nuclei with 2, 8, 20, 28, 50, or 82 protons or 2, 8, 20, 28, 50, 82, or 126 neutrons; results in very stable nuclei Nuclei wit ...
... Radioactive series (or nuclear disintegration series): a series of nuclear reactions that begins with an unstable nucleus and terminates with a stable one Magic numbers: nuclei with 2, 8, 20, 28, 50, or 82 protons or 2, 8, 20, 28, 50, 82, or 126 neutrons; results in very stable nuclei Nuclei wit ...
experiment 8 radioactive decay of nuclei
... 4.28% mass 113. (Using the nuclear masses of 114.9041 and 112.9043 instead of the number of nucleons, 115 and 113, the chemical weight of 114.82 can be calculated.) If the indium is placed where there are many free neutrons with kinetic energies, on the order of 0.03 eV, both isotopes In-115 and In- ...
... 4.28% mass 113. (Using the nuclear masses of 114.9041 and 112.9043 instead of the number of nucleons, 115 and 113, the chemical weight of 114.82 can be calculated.) If the indium is placed where there are many free neutrons with kinetic energies, on the order of 0.03 eV, both isotopes In-115 and In- ...
(or radioactive isotopes).
... high enough to kill the cancerous cells, but as low as possible to minimise the harm to the patient. ...
... high enough to kill the cancerous cells, but as low as possible to minimise the harm to the patient. ...
Atomic Theory Handout CNS 8
... and the idea of "complementarity" -- that things may have a dual nature (as the electron is both particle and wave) but we can only experience one aspect at a time. ...
... and the idea of "complementarity" -- that things may have a dual nature (as the electron is both particle and wave) but we can only experience one aspect at a time. ...
Mass-Energy Equivalence - Dr. Haleys Physics Class
... four new nuclei and cause fission reactions that release eight neutrons. The number of neutrons increases rapidly. The increasing number of neutrons causes more nuclei to have fission reactions and enormous energy is released. ...
... four new nuclei and cause fission reactions that release eight neutrons. The number of neutrons increases rapidly. The increasing number of neutrons causes more nuclei to have fission reactions and enormous energy is released. ...
Mass-Energy Equivalence
... four new nuclei and cause fission reactions that release eight neutrons. The number of neutrons increases rapidly. The increasing number of neutrons causes more nuclei to have fission reactions and enormous energy is released. ...
... four new nuclei and cause fission reactions that release eight neutrons. The number of neutrons increases rapidly. The increasing number of neutrons causes more nuclei to have fission reactions and enormous energy is released. ...
physics - Keith E. Holbert
... A=N+Z: atomic mass number (the number of nucleons). Definitions and Distinctions • atomic mass number (A) [integer number] vs. atomic weight or atomic mass (M) [real value]: M≅A • isotope: nuclides with equal number of protons, but different numbers of neutrons • isotone: nuclides with equal number ...
... A=N+Z: atomic mass number (the number of nucleons). Definitions and Distinctions • atomic mass number (A) [integer number] vs. atomic weight or atomic mass (M) [real value]: M≅A • isotope: nuclides with equal number of protons, but different numbers of neutrons • isotone: nuclides with equal number ...
Nuclear Chemistry
... • If you analyze a nuclear reaction & observe the products, you can determine the type of reaction that took place: 1- If both the mass number & atomic number decrease, alpha decay occurred. 2- If only the atomic number increases, beta decay has occurred. 3- If neither mass number or atomic number c ...
... • If you analyze a nuclear reaction & observe the products, you can determine the type of reaction that took place: 1- If both the mass number & atomic number decrease, alpha decay occurred. 2- If only the atomic number increases, beta decay has occurred. 3- If neither mass number or atomic number c ...
Chapter 21 Nuclear Chemistry - Ocean County Vocational
... • If you analyze a nuclear reaction & observe the products, you can determine the type of reaction that took place: 1- If both the mass number & atomic number decrease, alpha decay occurred. 2- If only the atomic number increases, beta decay has occurred. 3- If neither mass number or atomic number c ...
... • If you analyze a nuclear reaction & observe the products, you can determine the type of reaction that took place: 1- If both the mass number & atomic number decrease, alpha decay occurred. 2- If only the atomic number increases, beta decay has occurred. 3- If neither mass number or atomic number c ...
Energy per nucleon
... Fusion in stars • Stars convert hydrogen to helium and heavier elements. When Fe and Ni are reached, fusion stops. The star has burnt its nuclear fuel and collapses under its own gravity. • In massive stars, this collapse releases a huge amount of gravitational energy that leads to a supernova.The ...
... Fusion in stars • Stars convert hydrogen to helium and heavier elements. When Fe and Ni are reached, fusion stops. The star has burnt its nuclear fuel and collapses under its own gravity. • In massive stars, this collapse releases a huge amount of gravitational energy that leads to a supernova.The ...
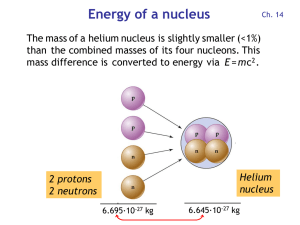
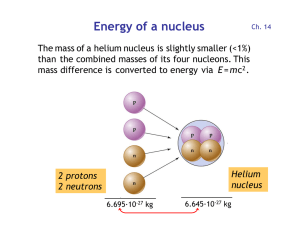
Energy of a nucleus
... Fusion in stars • Stars convert hydrogen to helium and heavier elements. When Fe and Ni are reached, fusion stops. The star has burnt its nuclear fuel and collapses under its own gravity. • In massive stars, this collapse releases a huge amount of gravitational energy that leads to a supernova.The ...
... Fusion in stars • Stars convert hydrogen to helium and heavier elements. When Fe and Ni are reached, fusion stops. The star has burnt its nuclear fuel and collapses under its own gravity. • In massive stars, this collapse releases a huge amount of gravitational energy that leads to a supernova.The ...
Chapter 14 section 2
... particle. Some eject an electron called a beta particle. A beta particle is a high-energy electron that comes from the nucleus, not the electron cloud. But, the nucleus contains only protons and neutrons. How can it give off an electron? In this kind of transmutation, a neutron becomes unstable. It ...
... particle. Some eject an electron called a beta particle. A beta particle is a high-energy electron that comes from the nucleus, not the electron cloud. But, the nucleus contains only protons and neutrons. How can it give off an electron? In this kind of transmutation, a neutron becomes unstable. It ...
Elements!
... • Atomic Model has changed dramatically through history •Subatomic particles make up all atoms ...
... • Atomic Model has changed dramatically through history •Subatomic particles make up all atoms ...
Document
... In this reaction two light atomic nuclei, when they are very close to each other, fuse together to form a single heavier nucleus of a new element. The process is exothermic (release of energy). The nuclear fusions occur at only very high temperatures. When 2 hydrogen nuclei fuse together by nuclear ...
... In this reaction two light atomic nuclei, when they are very close to each other, fuse together to form a single heavier nucleus of a new element. The process is exothermic (release of energy). The nuclear fusions occur at only very high temperatures. When 2 hydrogen nuclei fuse together by nuclear ...
t 1/2
... Figure 13.1b: Apparatus used by J. J. Thomson (1897) to measure the charge-to-mass ratio of the electron. The evacuated tube is similar to a TV picture tube. The negatively charged particles emitted from the cathode are deflected by either an electric field or a magnetic field. The parallel plates ...
... Figure 13.1b: Apparatus used by J. J. Thomson (1897) to measure the charge-to-mass ratio of the electron. The evacuated tube is similar to a TV picture tube. The negatively charged particles emitted from the cathode are deflected by either an electric field or a magnetic field. The parallel plates ...
Mass of individual atoms
... is determined by ratio of neutron to protons. Too many or too few neutrons makes an atom unstable. ...
... is determined by ratio of neutron to protons. Too many or too few neutrons makes an atom unstable. ...
U - Earth and Environmental Sciences
... Uranium-238 is abundant in the nuclear "fuel"; this leads to the production of plutonium. Nuclear fusion has the potential to yield enormous amounts of energy if it can be done in a controlled, sustained way. Consider the mass-to-energy numbers... 2 protons = 2 x 1.00728 u = 2.01456 u 2 neutrons = 2 ...
... Uranium-238 is abundant in the nuclear "fuel"; this leads to the production of plutonium. Nuclear fusion has the potential to yield enormous amounts of energy if it can be done in a controlled, sustained way. Consider the mass-to-energy numbers... 2 protons = 2 x 1.00728 u = 2.01456 u 2 neutrons = 2 ...
Radioactivityunit6
... 2. Divide 37.5 by 2 to get 18.75… so after two half lives you’ve got 18.75g. 3. Divide 18.75 by 2 to get 9.375. After three half lives have passed you’ve got 9.375g left. That’s pretty close to the 9.3g in the question, so after just a little more than three half lives you should have 9.3g left ove ...
... 2. Divide 37.5 by 2 to get 18.75… so after two half lives you’ve got 18.75g. 3. Divide 18.75 by 2 to get 9.375. After three half lives have passed you’ve got 9.375g left. That’s pretty close to the 9.3g in the question, so after just a little more than three half lives you should have 9.3g left ove ...
Radiation and Radioactive Decay
... nuclear force, thus the binding energy increases. As we pass iron, we again have even more nucleons, however the distance between them is increasing and the mutual attraction due to the (short-range) strong nuclear force is weakened. But the proton pairs are still feeling the electromagnet repulsion ...
... nuclear force, thus the binding energy increases. As we pass iron, we again have even more nucleons, however the distance between them is increasing and the mutual attraction due to the (short-range) strong nuclear force is weakened. But the proton pairs are still feeling the electromagnet repulsion ...