Chapter 28 for Chem
... c is the speed of light and c2 is a HUGE number. So even a little bit of mass can convert to a LOT of energy. ...
... c is the speed of light and c2 is a HUGE number. So even a little bit of mass can convert to a LOT of energy. ...
File
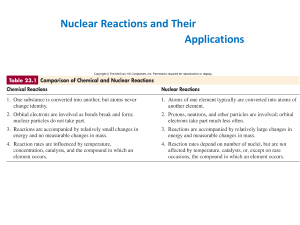
... • Atomic Number-the number of protons in the nucleus of an atom of a particular element. • Chemical Formula-an expression of the elements in a compound and their ratios in which the elements are denoted by their chemical ...
... • Atomic Number-the number of protons in the nucleus of an atom of a particular element. • Chemical Formula-an expression of the elements in a compound and their ratios in which the elements are denoted by their chemical ...
File
... The C-14 is oxidized to CO2, which circulates through the biosphere. When a plant dies, the C-14 is not replenished. But the C-14 continues to decay with t1/2 = 5730 years. Activity of a sample can be used to date the sample. ...
... The C-14 is oxidized to CO2, which circulates through the biosphere. When a plant dies, the C-14 is not replenished. But the C-14 continues to decay with t1/2 = 5730 years. Activity of a sample can be used to date the sample. ...
document
... first to form a new nucleus Work out what the new nucleus is Find the nucleus on your graph and add it in Join the points with an arrow ...
... first to form a new nucleus Work out what the new nucleus is Find the nucleus on your graph and add it in Join the points with an arrow ...
Revision of Atomic Structure and Nuclide Notations Nuclide
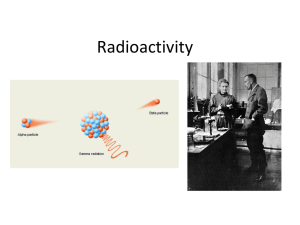
... The atoms of most elements have isotopes. Some of these isotopes have nuclei which are unstable and give out different types of particles or rays. This release (emission) of particles and rays is what we call radiation and helps to make the atom more stable. Radioactivity is all around us. We are co ...
... The atoms of most elements have isotopes. Some of these isotopes have nuclei which are unstable and give out different types of particles or rays. This release (emission) of particles and rays is what we call radiation and helps to make the atom more stable. Radioactivity is all around us. We are co ...
Nuclear Physics SL - Hockerill Students
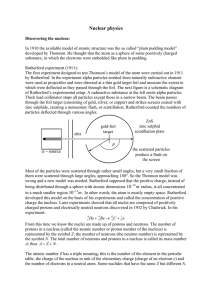
... It was also discovered by JJ Thompson and others that atoms of the same element could have different ...
... It was also discovered by JJ Thompson and others that atoms of the same element could have different ...
Abstract
... where SMOW is standard mean ocean water that is used as a standard material for oxygen isotope ratios. Isotope ratios change during physico-chemical and nuclear reactions. In the physico-chemical reactions, mass differences between isotopes are responsible for changing isotope ratios, because heavie ...
... where SMOW is standard mean ocean water that is used as a standard material for oxygen isotope ratios. Isotope ratios change during physico-chemical and nuclear reactions. In the physico-chemical reactions, mass differences between isotopes are responsible for changing isotope ratios, because heavie ...
chapter 21 blm answer key
... In fission, a large nucleus splits into two smaller nuclei. In fusion, two small nuclei come together to form one larger nucleus. In both cases, the binding energy per nucleon of the product(s) is larger than the binding energy per nucleon of the reactant(s). This means that the total mass of the pr ...
... In fission, a large nucleus splits into two smaller nuclei. In fusion, two small nuclei come together to form one larger nucleus. In both cases, the binding energy per nucleon of the product(s) is larger than the binding energy per nucleon of the reactant(s). This means that the total mass of the pr ...
Balancing a Nuclear Equation
... • An unstable nucleus spontaneously gives off radiation to become more ...
... • An unstable nucleus spontaneously gives off radiation to become more ...
Structure of the nucleus • It is now known that the nucleus consists of
... Einstein suggested that mass and energy are equivalent, and that when there was a decrease in mass, an equivalent amount of energy was produced. ...
... Einstein suggested that mass and energy are equivalent, and that when there was a decrease in mass, an equivalent amount of energy was produced. ...
NAME GRADED: LET IT BEGIN!!! ____ / 30 pts DIRECTIONS: Use
... Necessary Background: When an isotope is a nuclear radioactive isotope, it means that it can spontaneously breakdown, by emitting alpha particles (effectively He-4 nuclei each equaling 2 protons and 2 neutrons, and of course, 0 electrons), beta particles (high speed e- from degenerating neutrons) or ...
... Necessary Background: When an isotope is a nuclear radioactive isotope, it means that it can spontaneously breakdown, by emitting alpha particles (effectively He-4 nuclei each equaling 2 protons and 2 neutrons, and of course, 0 electrons), beta particles (high speed e- from degenerating neutrons) or ...
Nuclear physics α −
... number of neutrons. In several cases the nucleus is sable structure. What kind of interaction exists inside the nucleus? This interaction is not gravitational (the gravitational interaction is very week), not electrical interaction (the neutrons are neutral, and what is more the protons repulse each ...
... number of neutrons. In several cases the nucleus is sable structure. What kind of interaction exists inside the nucleus? This interaction is not gravitational (the gravitational interaction is very week), not electrical interaction (the neutrons are neutral, and what is more the protons repulse each ...
Chapter 10
... • Remember for section 2.2, this defines an isotope of boron. • In nuclear chemistry this is often called a nuclide. • This is not the only isotope (nuclide) of boron. – boron-10 also exists – How many protons and neutrons does boron-10 have? – 5 protons, 5 neutrons ...
... • Remember for section 2.2, this defines an isotope of boron. • In nuclear chemistry this is often called a nuclide. • This is not the only isotope (nuclide) of boron. – boron-10 also exists – How many protons and neutrons does boron-10 have? – 5 protons, 5 neutrons ...
Radioactivity
... Radioactive Decay • Radioisotopes decay from one element to another until they are transformed into stable, non-radioactive isotopes. • For example, 238U decays 11 times, shedding mass and energy each time, eventually becoming 206Pb, a stable isotope. • Radioactive decay is spontaneous – it does no ...
... Radioactive Decay • Radioisotopes decay from one element to another until they are transformed into stable, non-radioactive isotopes. • For example, 238U decays 11 times, shedding mass and energy each time, eventually becoming 206Pb, a stable isotope. • Radioactive decay is spontaneous – it does no ...
Lecture14
... Nuclear Physics Nuclear Transformations • the nuclear structure of atoms is changed by three different mechanisms: – fission: splitting of heavy nuclei into two lighter ones with the releases of neutrons and energy • spontaneous • neutron-induced ...
... Nuclear Physics Nuclear Transformations • the nuclear structure of atoms is changed by three different mechanisms: – fission: splitting of heavy nuclei into two lighter ones with the releases of neutrons and energy • spontaneous • neutron-induced ...
Matter and Energy
... composition. Examples include splitting wood, mixing cake batter ingredients, and shaping metal. Chemical – involves a change to the chemical composition of a substance. Examples include burning wood, baking cake batter, and metal rusting. Nuclear – certain isotopes are so unstable they are able to ...
... composition. Examples include splitting wood, mixing cake batter ingredients, and shaping metal. Chemical – involves a change to the chemical composition of a substance. Examples include burning wood, baking cake batter, and metal rusting. Nuclear – certain isotopes are so unstable they are able to ...
Examination 1
... Up to this point all combination of chemical species in a chemical reaction involved only a breaking of bonds within the molecules that were reaction to form new bonds and new molecules. The identity of the atoms participating in the reaction remained the same - so in balancing the reaction we alway ...
... Up to this point all combination of chemical species in a chemical reaction involved only a breaking of bonds within the molecules that were reaction to form new bonds and new molecules. The identity of the atoms participating in the reaction remained the same - so in balancing the reaction we alway ...
Nuclear Chemistry
... Up to this point all combination of chemical species in a chemical reaction involved only a breaking of bonds within the molecules that were reaction to form new bonds and new molecules. The identity of the atoms participating in the reaction remained the same - so in balancing the reaction we alway ...
... Up to this point all combination of chemical species in a chemical reaction involved only a breaking of bonds within the molecules that were reaction to form new bonds and new molecules. The identity of the atoms participating in the reaction remained the same - so in balancing the reaction we alway ...
Syllabus overview
... Students should be familiar with the concept of thermal equilibrium. 3.1.2 State the relation between the Kelvin and Celsius scales of temperature. T/K = t/°C + 273 is sufficient. 3.1.3 State that the internal energy of a substance is the total potential energy and random kinetic energy of the molec ...
... Students should be familiar with the concept of thermal equilibrium. 3.1.2 State the relation between the Kelvin and Celsius scales of temperature. T/K = t/°C + 273 is sufficient. 3.1.3 State that the internal energy of a substance is the total potential energy and random kinetic energy of the molec ...
Chapter 25
... In 1988 Kai Hansen left his band Helloween since he was tired of the bad atmosphere in and around the band. Together with Dirk Schlächter and Ralf Scheepers he formed a new band called Gamma Ray. Who will have them on iPod by the end of this unit?? ...
... In 1988 Kai Hansen left his band Helloween since he was tired of the bad atmosphere in and around the band. Together with Dirk Schlächter and Ralf Scheepers he formed a new band called Gamma Ray. Who will have them on iPod by the end of this unit?? ...
Average Atomic Mass
... • Radioactive atoms undergo significant changes that can alter their identities. • They emit radiation because their nuclei are unstable. • They gain stability by losing energy when they emit radiation. • This process is done spontaneously and is known as radioactive decay. • They continue this proc ...
... • Radioactive atoms undergo significant changes that can alter their identities. • They emit radiation because their nuclei are unstable. • They gain stability by losing energy when they emit radiation. • This process is done spontaneously and is known as radioactive decay. • They continue this proc ...
ch10_sec1_rc
... • Gamma rays are high-energy electromagnetic radiation. • gamma ray: a high-energy photon emitted by a nucleus during fission and radioactive decay • When atoms decay by emitting a or b particles to form a new atom, the nuclei of the new atom formed may still have too much energy to be completely st ...
... • Gamma rays are high-energy electromagnetic radiation. • gamma ray: a high-energy photon emitted by a nucleus during fission and radioactive decay • When atoms decay by emitting a or b particles to form a new atom, the nuclei of the new atom formed may still have too much energy to be completely st ...
Nuclear Reactions - Socastee High School
... much higher than the light isotopes of hydrogen. • There is a trend for nuclides of nucleon numbers in multiples of 4 to be particularly stable (i.e. have a high binding energy). • Fe is the most stable nuclide. • The largest nuclides tend to be less stable, with slightly lower ...
... much higher than the light isotopes of hydrogen. • There is a trend for nuclides of nucleon numbers in multiples of 4 to be particularly stable (i.e. have a high binding energy). • Fe is the most stable nuclide. • The largest nuclides tend to be less stable, with slightly lower ...