1 + - crypt
... Define what is meant by nuclear fission. With aid of a copy of Figure 1, explain what is meant by a chain reaction. Explain the purpose of fission neutrons in a chain reaction. Copy and answer question (a) on page 202. What isotopes are used for fission reactions? Copy Figure 3 on page 203 explain h ...
... Define what is meant by nuclear fission. With aid of a copy of Figure 1, explain what is meant by a chain reaction. Explain the purpose of fission neutrons in a chain reaction. Copy and answer question (a) on page 202. What isotopes are used for fission reactions? Copy Figure 3 on page 203 explain h ...
Radioactivity Revision Questions Decay – Nucleus
... Sometimes the nucleus of an atom is unstable. A change will occur in the nucleus to make it more stable. The change is called a decay 2. During Radioactive Decay, what can a Nucleus Emit? When a nucleus decays it will emit (give out) some particles or waves. Emitting particles or waves from the nucl ...
... Sometimes the nucleus of an atom is unstable. A change will occur in the nucleus to make it more stable. The change is called a decay 2. During Radioactive Decay, what can a Nucleus Emit? When a nucleus decays it will emit (give out) some particles or waves. Emitting particles or waves from the nucl ...
Grade 10S Physics T3W5 material
... Radioactive labeling can be used in crime detection. 5‐ By measuring in the rocks the present rate of radioactivity or the concentration of the non radioactive products of radioactivity, the age of the rock can be determined. 6‐ a. Lower doses may cause Leukemia within 2years or other forms of ca ...
... Radioactive labeling can be used in crime detection. 5‐ By measuring in the rocks the present rate of radioactivity or the concentration of the non radioactive products of radioactivity, the age of the rock can be determined. 6‐ a. Lower doses may cause Leukemia within 2years or other forms of ca ...
nuclear force
... •All elements beyond bismuth on the periodic table are unstable and undergo some sort of ‘decay’ in order to ...
... •All elements beyond bismuth on the periodic table are unstable and undergo some sort of ‘decay’ in order to ...
Content Domain III: Chemistry—Atomic Theory and
... Language (Atom) – atomic mass, atomic number, electron cloud, electron, ion, isotopes, law of conservation of matter, matter, neutron, nucleus, proton, alpha particles, beta particles, fission, fusion, gamma radiation, half-life, radioactive decay. The current model suggests that an atom consists of ...
... Language (Atom) – atomic mass, atomic number, electron cloud, electron, ion, isotopes, law of conservation of matter, matter, neutron, nucleus, proton, alpha particles, beta particles, fission, fusion, gamma radiation, half-life, radioactive decay. The current model suggests that an atom consists of ...
Nuclear Chemistry
... • Example: Beta decay process is the decay of iodine- 131 into xenon131 by beta-particle emission • The mass number of the product nucleus is the same as that of the original nucleus ( they are both 131), but its atomic number has increased by 1 (54 instead of 53). This changed in atomic number, and ...
... • Example: Beta decay process is the decay of iodine- 131 into xenon131 by beta-particle emission • The mass number of the product nucleus is the same as that of the original nucleus ( they are both 131), but its atomic number has increased by 1 (54 instead of 53). This changed in atomic number, and ...
Notes for the Structure of Atoms (Chapter 4, Sect
... neutrons relative to other atoms of the same element. They will have a different mass because the number of neutrons vary. ...
... neutrons relative to other atoms of the same element. They will have a different mass because the number of neutrons vary. ...
Radioactivity Notes Day 1 and 2 Apr 23 and Apr 24
... Radioactivity and Its History • Radiation is everywhere, but can be difficult to detect. ...
... Radioactivity and Its History • Radiation is everywhere, but can be difficult to detect. ...
FYS 3520-Midterm2014
... h) What is the heaviest (largest Z and largest A) nucleus we have managed to produce so far (do not use the textbook) i) Which element Z has the highest number of stable isotopes? Why? j) Is it harder to steal a neutron from 208Pd or 11Li or 4He? k) Why was Rutherford amazed by the results of his fa ...
... h) What is the heaviest (largest Z and largest A) nucleus we have managed to produce so far (do not use the textbook) i) Which element Z has the highest number of stable isotopes? Why? j) Is it harder to steal a neutron from 208Pd or 11Li or 4He? k) Why was Rutherford amazed by the results of his fa ...
Chem vocab quiz definitons
... Valence electrons are the electrons in the outer shell and control the elements reactivity. Molecule is the smallest particle of a compound that has all the properties of the compound. Compound is a pure substance that is made of two or more elements chemically bound together. Pure substances are bo ...
... Valence electrons are the electrons in the outer shell and control the elements reactivity. Molecule is the smallest particle of a compound that has all the properties of the compound. Compound is a pure substance that is made of two or more elements chemically bound together. Pure substances are bo ...
Unit 2: The Atom
... Beta Decay •Beta decay is how elements who have too many neutrons try to become stable (on top of the band) •Beta reactions will always have ß or e- on the right side! ...
... Beta Decay •Beta decay is how elements who have too many neutrons try to become stable (on top of the band) •Beta reactions will always have ß or e- on the right side! ...
Radioactivity
... • Mass to energy conversion is governed by DE = Dmc2, where c = the speed of light in a vacuum (3.0x108m/s) • Nuclear binding energy is the energy lost when the nucleus is formed. • Mass equivalent of the nuclear binding energy is the mass defect. • Protons and neutrons in the nucleus have less mass ...
... • Mass to energy conversion is governed by DE = Dmc2, where c = the speed of light in a vacuum (3.0x108m/s) • Nuclear binding energy is the energy lost when the nucleus is formed. • Mass equivalent of the nuclear binding energy is the mass defect. • Protons and neutrons in the nucleus have less mass ...
Radioactivity
... • Mass to energy conversion is governed by DE = Dmc2, where c = the speed of light in a vacuum (3.0x108m/s) • Nuclear binding energy is the energy lost when the nucleus is formed. • Mass equivalent of the nuclear binding energy is the mass defect. • Protons and neutrons in the nucleus have less mass ...
... • Mass to energy conversion is governed by DE = Dmc2, where c = the speed of light in a vacuum (3.0x108m/s) • Nuclear binding energy is the energy lost when the nucleus is formed. • Mass equivalent of the nuclear binding energy is the mass defect. • Protons and neutrons in the nucleus have less mass ...
Name
... a. Radiotherapy is a treatment that uses controlled doses of nuclear radiation for treating diseases such as cancer 4. Agriculture uses radioactive tracers and radio-isotopes a. On research farms, radioactive tracers help scientists to understand biochemical processes in plants D. Possible risks of ...
... a. Radiotherapy is a treatment that uses controlled doses of nuclear radiation for treating diseases such as cancer 4. Agriculture uses radioactive tracers and radio-isotopes a. On research farms, radioactive tracers help scientists to understand biochemical processes in plants D. Possible risks of ...
Nuclear Chemistry
... Two small nuclei can combine and release large amounts of energy To occur, the nuclei must be in an environment with high temperature. ...
... Two small nuclei can combine and release large amounts of energy To occur, the nuclei must be in an environment with high temperature. ...
Topic 13.2 Nuclear Physics
... • For elements with half-lives of the order of seconds, the ionisation properties of the radiations can be used. If the sample is placed in a tube across which an electric field is applied, the radiation from the source will ionise the air in the tube and thereby give rise to an ionisation current. ...
... • For elements with half-lives of the order of seconds, the ionisation properties of the radiations can be used. If the sample is placed in a tube across which an electric field is applied, the radiation from the source will ionise the air in the tube and thereby give rise to an ionisation current. ...
IB-ATOMIC-AND-NUCLEAR-PHYSICS-DEFINITIONS
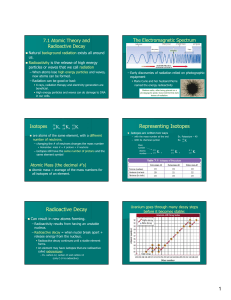
... NUCLEON: The particles in the nucleus (proton or neutron). NUCLEON NUMBER, A: The number of nucleons in the nucleus. PROTON NUMBER, Z: The number of protons in the nucleus. NEUTRON NUMBER, N: N=A-Z RADIOACTIVE DECAY: A random and spontaneous process in which unstable nuclei emit a particle (disinteg ...
... NUCLEON: The particles in the nucleus (proton or neutron). NUCLEON NUMBER, A: The number of nucleons in the nucleus. PROTON NUMBER, Z: The number of protons in the nucleus. NEUTRON NUMBER, N: N=A-Z RADIOACTIVE DECAY: A random and spontaneous process in which unstable nuclei emit a particle (disinteg ...
File
... identified by their mass, which is the total number of protons and neutrons. There are two ways that isotopes are generally written. They both use the mass of the atom where mass = (number of protons + number of neutrons). The first way is to put the mass as a superscript before the symbol of the el ...
... identified by their mass, which is the total number of protons and neutrons. There are two ways that isotopes are generally written. They both use the mass of the atom where mass = (number of protons + number of neutrons). The first way is to put the mass as a superscript before the symbol of the el ...
Lesson 13: Nuclear Propulsion Basics
... • However, in solid fuel they can only travel a microscopic distance, so their energy becomes converted into heat. • The balance of the energy comes from gamma rays emitted during or immediately following the fission process and from the kinetic energy of the neutrons. – Some of the latter are immed ...
... • However, in solid fuel they can only travel a microscopic distance, so their energy becomes converted into heat. • The balance of the energy comes from gamma rays emitted during or immediately following the fission process and from the kinetic energy of the neutrons. – Some of the latter are immed ...
Chapter 3
... - measure of how long the pollutant stays in the air, water, soil, or body. Classification of Pollutants: 1. Degradable (reduced to acceptable levels) 2. Slowly Degradable (decades or longer-DDT) 3. Nondegradable (natural processes cannot break down -lead, arsenic) ...
... - measure of how long the pollutant stays in the air, water, soil, or body. Classification of Pollutants: 1. Degradable (reduced to acceptable levels) 2. Slowly Degradable (decades or longer-DDT) 3. Nondegradable (natural processes cannot break down -lead, arsenic) ...
APES-Chapter-3
... - measure of how long the pollutant stays in the air, water, soil, or body. Classification of Pollutants: 1. Degradable (reduced to acceptable levels) 2. Slowly Degradable (decades or longer-DDT) 3. Nondegradable (natural processes cannot break down -lead, arsenic) ...
... - measure of how long the pollutant stays in the air, water, soil, or body. Classification of Pollutants: 1. Degradable (reduced to acceptable levels) 2. Slowly Degradable (decades or longer-DDT) 3. Nondegradable (natural processes cannot break down -lead, arsenic) ...
Chemistry: Nuclear Reactions Guided Inquiry + n → + + 3 n +
... Nuclear reactions are reactions that affect the nucleus of an atom. In nature, unstable nuclei undergo nuclear reactions to form more stable nuclei. Stable nuclei can also undergo nuclear reactions if ...
... Nuclear reactions are reactions that affect the nucleus of an atom. In nature, unstable nuclei undergo nuclear reactions to form more stable nuclei. Stable nuclei can also undergo nuclear reactions if ...
Half-life and Radioactive Decay guided notes
... and two neutrons released from the nucleus of the radioactive atom. This means that alpha particles have a ...
... and two neutrons released from the nucleus of the radioactive atom. This means that alpha particles have a ...