* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Nuclear Radiation1516
Nuclear fusion wikipedia , lookup
Isotopic labeling wikipedia , lookup
Fallout shelter wikipedia , lookup
Nuclear and radiation accidents and incidents wikipedia , lookup
Background radiation wikipedia , lookup
Nuclear fusion–fission hybrid wikipedia , lookup
Nuclear binding energy wikipedia , lookup
Ionizing radiation wikipedia , lookup
Nuclear fission wikipedia , lookup
Technetium-99m wikipedia , lookup
Nuclear fission product wikipedia , lookup
Radioactive decay wikipedia , lookup
Valley of stability wikipedia , lookup
Atomic nucleus wikipedia , lookup
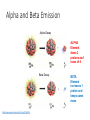
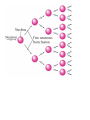
Nuclear Chemistry “Nuclear radiation is in the form of elementary particles emitted by an atomic nucleus, as alpha rays, beta rays, or gamma rays, produced by decay of radioactive substances or by nuclear fission.” Absorption of Radiation = alpha particle Weak energy = beta particle Moderate energy = gamma radiation High energy Timberlake, Chemistry 7th Edition, page 84 Characteristics of Some Ionizing Radiations Characteristics of Some Ionizing Radiation Property Alpha radiation Beta radiation Composition Alpha particle (helium nucleus) Beta particle (electron) High-energy electromagnetic radiation Symbol , He-4 , e Charge 2+ 1- 0 Mass (amu) 4 1/ 0 Common source Radium-226 Carbon-14 Cobalt-60 Approximate energy 5 MeV* 0.05 to 1 MeV 1 MeV Penetrating power Low (0.05 mm body tissue) Moderate (4 mm body tissue) Very high (penetrates body easily) Shielding Paper, clothing Metal foil *(1 MeV = 1.60 x 10-13 J) Gamma radiation 1837 https://www.youtube.com/watch?v=uJ3ea9fa6CA Lead, concrete (incompletel shields) RADIOACTIVITY Spontaneous emission of particles (alpha, beta, neutron) or radiation (gamma), or both at the same time, from the decay of certain radioisotopes. Isotopes of some elements are radioactive, especially the large elements. Radioactive elements are unstable and will undergo radioactive decay. These radioactive elements release particles (alpha and beta) in order to stabilize. Radioactive decay results in the release of particles and the formation of an isotope of a new element. In additions to particles being released, sometimes gamma radiation is released. HALF LIFE • Amount of time it takes to decay ½ of a sample. • Varies from milliseconds to billions of years. This is always the slope on a half life graph • Start with 1.6 g of 187Er with a half-life of 400 seconds. • How many grams after 3 half-lifes? • How much time does it take to have 0.025 grams? • How many half-lifes to get to 0.00625? HALF LIFE Number of Mass half life Time s % Fraction/ ratio 0 1.6 0 100 1 1 0.8 400 50 .5 2 0.4 800 25 .25 3 0.2 1200 12.5 .125 4 0.1 1600 6.25 .0625 5 0.05 2000 3.125 .03125 6 0.025 2400 1.5625 .015625 You can make a chart to determine the mass, number of half life, time, percent and fraction remaining. Number of half life, %, and Fraction are the same on every chart. HALF LIFE 1.Number 2.Mass of half life 3.Time s 4.% 5.Fraction /ratio 0 1.6 0 100 1 1 0.8 400 50 .5 2 0.4 800 25 .25 3 0.2 1200 12.5 .125 4 0.1 1600 6.25 .0625 5 0.05 2000 3.125 .03125 6 0.025 2400 1.5625 .015625 How to fill in the chart 1.Number of half lifeadd 1 to each row. 2.Mass-Divide each row’s mass by 2 3.Time-Add time to each row. 4.%- always start with 100 at 0 time and divide each row by 2. 5. Fraction or ratioalways start with 1 and divide each row by 2. HALF LIFE A radioisotope will decay ½ of the mass of its sample during the first half life. The mass of the radioactive sample is ½ of the starting mass. The mass of the nonradioactive sample has increased. What is the half life of the sample? If the starting mass was 10 g, how many g remain after 30 years? How many years will it take to reduce the sample to 0.078 g? ALPHA DECAY • Alpha decay is the process of releasing an alpha particle (He nucleus) from the nucleus of a radioisotope. • The result of alpha decay is a reduction of the mass number by 4 and the atomic number by 2 • The equation is written using isotope notation. • Both sides of the arrow are equal in mass and number of protons http://www.youtube.com/watch?v=J8p7OIdyt54 Release of an alpha particle, He nucleus. Alpha Decay U He 238 92 4 2 2 234 90 Th https://www.youtube.com/watch?v=oFdR_yMKOCw Timberlake, Chemistry 7th Edition, page 87 ALPHA DECAY 240 94 All numbers are equal on each side of the arrow. 240 240 94 94 The addition of the mass numbers on the left and right of the arrow are equal. The addition of the atomic numbers on the left and right of the arrow are equal. Pu 236U + 4He 92 u 2 BETA DECAY 56 The product of this decay is an atom with the same mass but 1 more proton and an electron. • Beta decay is the release of a beta particle (electron) from the nucleus of an atom. • WHAT??? How does an electron come out of the nucleus if they are in the energy levels??? • Are you ready for this? A neutron changes into a proton and an electron. After all, a positive and a negative make it neutral. Beta Decay Changes a neutron into a proton and an electron. The electron is released as a beta particle. Timberlake, Chemistry 7th Edition, page 90 Alpha and Beta Emission Alpha Decay ALPHA Element down 2 protons and mass of 4 Beta Decay https://www.youtube.com/watch?v=o-9yt7OAYmE BETA Element increases 1 proton and keeps same mass Alpha and Beta Emission BETA PLUS DECAY • The symbol for a positron is e+. • PET-positron emission tomography is a scan used to find cancer. • Are you sitting down? Now we have a POSITIVE electron. It is called a positron. Positron decay is like a mirror image of beta decay. 1) A proton to become a neutron. 2) It emits a positron 3) The atomic number goes DOWN by one and mass number remains unchanged. ELECTRON CAPTURE • 3) The atomic number goes down by 1 and mass number stays the same. • Electron capture is not like any other decay alpha, beta, or position. All other decays shoot something out of the nucleus. • 1) An electron from the closest energy level falls into the nucleus, which causes a proton to become a neutron. • 2) A neutrino(neutral particle with no mass) is emitted from the nucleus. IMPORTANT: MASS AND CHARGE = ON BOTH SIDES OF ARROW! 4+10 = 13+1 2+5 = 7+0 New Radioactive Isotope Timberlake, Chemistry 7th Edition, page 92 NUCLEAR FISSION Nuclear Fission is the splitting of a radioactive isotope by bombardment with neutrons so that the atom splits into smaller elements with a release of energy. NUCLEAR FISSION When a nucleus fissions, it splits into several smaller fragments. These fragments, or fission products, are about equal to half the original mass. Two or three neutrons are also emitted. The sum of the masses of these fragments is less than the original mass. This 'missing' mass (about 0.1 percent of the original mass) has been converted into energy according to Einstein's equation. Fission can occur when a nucleus of a heavy atom captures a neutron, or it can happen spontaneously. Fissionable U-235 • A nuclear chain reaction occurs when one nuclear reaction causes an average of one or more nuclear reactions, thus leading to a self-propagating number of these reactions. The nuclear reaction is the fission of heavy isotopes. • The nuclear chain reaction is unique since it releases several million times more energy per reaction than any chemical reaction. http://www.youtube.com/watch?v=FQGtpo2IUxA&NR=1&feature=fvwp • https://www.youtube.com/watch?v=0v8i4v1mieU http://www.youtube.com/watch?v=Pmy5fivI_4U Chain reaction Fission Process NUCLEAR POWER PLANTS Electricity in Illinois is about 50% coal generated and 50% nuclear power generated. NULCEAR POWER ILLINOIS Nuclear Reactor https://www.youtube.com/watch?v=rBvUtY0PfB8 Nuclear Power Debate Cons • Radiation exposure possible from accident or terrorist • No where safe to bury waste • Moving waste a potential danger • Thermal pollution • Long time to build nuclear plants • U will be depleted • https://www.youtube.com/watch?v=BdbitRlbLDc • https://www.youtube.com/watch?v=AkHLQfe3dw4 https://www.youtube.com/watch?v=VJfIbBDR3e8 Pros No greenhouse gases Very little solid waste Less extensive mining to extract U Less air pollution Generate large amounts of electricity from a single plant Nuclear Fusion + 1 4H 1 4 H nuclei + 0 2 e -1 2 electrons 4 1 He2 HeEnergy + FISSION FUSION • Splits atoms • Combines atoms • Releases radiation • No radiation • Used in nuclear power plants • On the sun FISSION AND FUSION • Release of large amounts of energy • Changes in the nuclei of atoms FISSION VS. FUSION • • • • • • • • • • • detect leaks in pipelines. attacking abnormal cells or harmful bacteria. treat cancer preserve food sterilize medical instruments tracer in medical research studies of human metabolism. Military home smoke detectors. calculate the age of mineral Pest control OTHER USES FOR RADIOISOTOPES