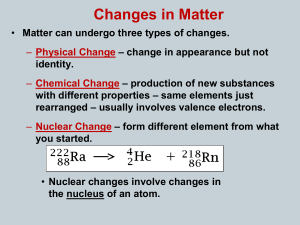
Chapter 9 Natural Radioactivity
... • In nuclear chemistry often called a nuclide • This is not the only isotope of boron – boron-10 also exists – How many protons and neutrons does boron-10 have? • 5 protons, 5 neutrons ...
... • In nuclear chemistry often called a nuclide • This is not the only isotope of boron – boron-10 also exists – How many protons and neutrons does boron-10 have? • 5 protons, 5 neutrons ...
AC Geophysical Science Study Guide
... 16. Compare the atomic mass of an atom with the atomic mass of the constituent particles that make up that mass. 17. What is binding energy? How do you determine binding energy? 18. The hydrogen isotope H-3 has a nuclear mass of 3.0155 µ, and the helium isotope He-3 has a nuclear mass of 3.0149 µ. F ...
... 16. Compare the atomic mass of an atom with the atomic mass of the constituent particles that make up that mass. 17. What is binding energy? How do you determine binding energy? 18. The hydrogen isotope H-3 has a nuclear mass of 3.0155 µ, and the helium isotope He-3 has a nuclear mass of 3.0149 µ. F ...
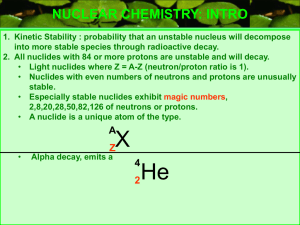
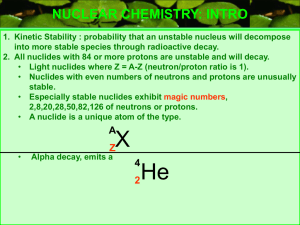
(neutron/proton ratio is 1).
... NUCLEAR CHEMISTRY: INTRO 1. Kinetic Stability : probability that an unstable nucleus will decompose into more stable species through radioactive decay. 2. All nuclides with 84 or more protons are unstable and will decay. • Light nuclides where Z = A-Z (neutron/proton ratio is 1). • Nuclides with eve ...
... NUCLEAR CHEMISTRY: INTRO 1. Kinetic Stability : probability that an unstable nucleus will decompose into more stable species through radioactive decay. 2. All nuclides with 84 or more protons are unstable and will decay. • Light nuclides where Z = A-Z (neutron/proton ratio is 1). • Nuclides with eve ...
NUCLEAR CHEMISTRY: INTRO
... NUCLEAR CHEMISTRY: INTRO 1. Kinetic Stability : probability that an unstable nucleus will decompose into more stable species through radioactive decay. 2. All nuclides with 84 or more protons are unstable and will decay. • Light nuclides where Z = A-Z (neutron/proton ratio is 1). • Nuclides with eve ...
... NUCLEAR CHEMISTRY: INTRO 1. Kinetic Stability : probability that an unstable nucleus will decompose into more stable species through radioactive decay. 2. All nuclides with 84 or more protons are unstable and will decay. • Light nuclides where Z = A-Z (neutron/proton ratio is 1). • Nuclides with eve ...
30.1 Radioactivity The atom is the smallest unit of achemical
... The atom is the smallest unit of achemical element, such as gold or carbon, which has the physical and chemical properties of the element. Nucleus Atoms have a dense center called a nucleus where you can find protons and neutrons. The nucleus is surrounded by orbiting electrons. A atomic nucleus is ...
... The atom is the smallest unit of achemical element, such as gold or carbon, which has the physical and chemical properties of the element. Nucleus Atoms have a dense center called a nucleus where you can find protons and neutrons. The nucleus is surrounded by orbiting electrons. A atomic nucleus is ...
The Band of Stability
... Protons may be converted to electrons by positron emission. A positron is the anti-particle of the electron. Example: ...
... Protons may be converted to electrons by positron emission. A positron is the anti-particle of the electron. Example: ...
File
... isotopes gain stability by undergoing changes and emitting large amounts of energy (radiation) • Nearly all isotopes with atomic number of 84 or greater are unstable ...
... isotopes gain stability by undergoing changes and emitting large amounts of energy (radiation) • Nearly all isotopes with atomic number of 84 or greater are unstable ...
Notes - Science With Horne
... isotopes gain stability by undergoing changes and emitting large amounts of energy (radiation) • Nearly all isotopes with atomic number of 84 or greater are unstable ...
... isotopes gain stability by undergoing changes and emitting large amounts of energy (radiation) • Nearly all isotopes with atomic number of 84 or greater are unstable ...
Physics: Principles and Applications, 6e Giancoli
... 19) An atom has 98 protons and 249 nucleons. If it undergoes alpha decay, what are the number of protons and neutrons, respectively, in the daughter nucleus? A) 100, 245 B) 94, 247 C) 96, 245 D) 100, 249 20) An element with atomic number 6 undergoes β- decay. Its atomic number is now A) 7. B) 6. C) ...
... 19) An atom has 98 protons and 249 nucleons. If it undergoes alpha decay, what are the number of protons and neutrons, respectively, in the daughter nucleus? A) 100, 245 B) 94, 247 C) 96, 245 D) 100, 249 20) An element with atomic number 6 undergoes β- decay. Its atomic number is now A) 7. B) 6. C) ...
Decommissioning a nuclear reactor
... Radioactive decay is the process by which an unstable isotope changes into another isotope of a different element, or into a less energetic form of the same isotope by emitting alpha, beta or gamma radiation. If the decay product is also unstable, decay continues until a stable product is reached. T ...
... Radioactive decay is the process by which an unstable isotope changes into another isotope of a different element, or into a less energetic form of the same isotope by emitting alpha, beta or gamma radiation. If the decay product is also unstable, decay continues until a stable product is reached. T ...
Chemistry Standard 2A-Nucleus Section 20.1
... 4. The stability of an isotope nucleus depends on the ____. Page answer is found ____________ a. valence electrons c. number of neutrons b. atomic number d. neutron-to-proton ratio 5. What is the process in which an unstable atomic nucleus emits charged particles or energy or both? Page answer is fo ...
... 4. The stability of an isotope nucleus depends on the ____. Page answer is found ____________ a. valence electrons c. number of neutrons b. atomic number d. neutron-to-proton ratio 5. What is the process in which an unstable atomic nucleus emits charged particles or energy or both? Page answer is fo ...
NASC 1110
... Nuclear reactions are changes of elemental composition in atomic interactions. In chemical reactions atomic composition does not change. Elements lighter than Fe can be produced by fusion of 2 smaller nuclei. This is possible, because binding energy increases with atomic number. ...
... Nuclear reactions are changes of elemental composition in atomic interactions. In chemical reactions atomic composition does not change. Elements lighter than Fe can be produced by fusion of 2 smaller nuclei. This is possible, because binding energy increases with atomic number. ...
Ch 10 Nuclear Chemistry
... • The greater the number of protons in a nucleus the greater is the electric force that repels those protons. • In larger nuclei, the repulsive electric force is stronger than in smaller nuclei • Larger numbers of electric forces make larger nucleus less stable ...
... • The greater the number of protons in a nucleus the greater is the electric force that repels those protons. • In larger nuclei, the repulsive electric force is stronger than in smaller nuclei • Larger numbers of electric forces make larger nucleus less stable ...
File
... phosphorus is true? A. Arsenic and phosphorus have the same number of valence electrons. B. The diameter of an arsenic atom is smaller than the diameter of a phosphorus atom. C. Because they are in the same group, the two elements have the same ionization energy. D. More energy is required to remove ...
... phosphorus is true? A. Arsenic and phosphorus have the same number of valence electrons. B. The diameter of an arsenic atom is smaller than the diameter of a phosphorus atom. C. Because they are in the same group, the two elements have the same ionization energy. D. More energy is required to remove ...
5.7 Nuclear Radiation
... – These changes are always accompanied by the emission of large amounts of energy. – Unlike chemical reactions, nuclear reactions are not affected by changes in temperature, pressure, or the presence of catalysts. – Nuclear reactions of given radioisotope cannot be slowed down, speeded up, or stopp ...
... – These changes are always accompanied by the emission of large amounts of energy. – Unlike chemical reactions, nuclear reactions are not affected by changes in temperature, pressure, or the presence of catalysts. – Nuclear reactions of given radioisotope cannot be slowed down, speeded up, or stopp ...
Chem 1721 Brief Notes: Chapter 20 Chapter 20: Nuclear Chemistry
... isotope symbols show the mass number superscripted in front of the element symbol; may or may not be combined with the atomic number subscripted in front of element symbol i.e. ...
... isotope symbols show the mass number superscripted in front of the element symbol; may or may not be combined with the atomic number subscripted in front of element symbol i.e. ...
By what process do most stars release energy? A. Electromagnetic
... The nal elements produced by radioactive decay di er from the original radioactive elements because the nuclei of the nal elements are always A. ...
... The nal elements produced by radioactive decay di er from the original radioactive elements because the nuclei of the nal elements are always A. ...
Nuclear Chemistry
... • Gamma rays are emitted during nuclear reactions, either alone or with other types of radiation • Gamma rays do NOT change the mass number or atomic number because they are energy not matter. ...
... • Gamma rays are emitted during nuclear reactions, either alone or with other types of radiation • Gamma rays do NOT change the mass number or atomic number because they are energy not matter. ...
Radioactivity - Teach Nuclear
... Produced when the nucleus of an atom is in an excited state and then releases energy, becoming more stable When a nucleus emits an or β particle, the daughter nucleus is sometimes left in an excited state. It can then jump down to a lower level by emitting a gamma ray ...
... Produced when the nucleus of an atom is in an excited state and then releases energy, becoming more stable When a nucleus emits an or β particle, the daughter nucleus is sometimes left in an excited state. It can then jump down to a lower level by emitting a gamma ray ...
Atomic Structure and Radioactivity
... - decay: the nucleus composition does not change. -decay occurs in large, unstable, nuclei. The heaviest stable isotope is 20983Bi. -decay transforms a neutron into a proton (n p+ + e ) Electron capture: p+ + e n and p+ n + e+ ...
... - decay: the nucleus composition does not change. -decay occurs in large, unstable, nuclei. The heaviest stable isotope is 20983Bi. -decay transforms a neutron into a proton (n p+ + e ) Electron capture: p+ + e n and p+ n + e+ ...
Half Life
... 13. If an original sample of carbon-14 weighs 10.0 grams and the half-life of carbon-14 is 5700 years, then at the end of 5700 years the amount of carbon-14 remaining will be a. 2.5 g b. 5.0 g c. 10.0 g d. 8.0 g 14. When an atom of uranium undergoes fission, the noble gas produced is a. argon b. neo ...
... 13. If an original sample of carbon-14 weighs 10.0 grams and the half-life of carbon-14 is 5700 years, then at the end of 5700 years the amount of carbon-14 remaining will be a. 2.5 g b. 5.0 g c. 10.0 g d. 8.0 g 14. When an atom of uranium undergoes fission, the noble gas produced is a. argon b. neo ...
nuclear chemistry - Magoffin County Schools
... pursue the creation of an atomic weapon. The result was the creation of two very powerful atomic bombs named FAT MAN and LITTLE BOY which were used against the cities of HIROSHIMA and NAGASAKI, JAPAN, effectively brining the World War II to an end. In this unit, we will explore the basic principles ...
... pursue the creation of an atomic weapon. The result was the creation of two very powerful atomic bombs named FAT MAN and LITTLE BOY which were used against the cities of HIROSHIMA and NAGASAKI, JAPAN, effectively brining the World War II to an end. In this unit, we will explore the basic principles ...
File
... A) Heavy nuclei split into lighter nuclei. B) Light nuclei form into heavier nuclei. C) Energy is released and less stable elements are formed. D) Energy is absorbed and more stable elements are formed. 30. When a nucleus with a high mass undergoes fission, the resulting nuclei are more stable than ...
... A) Heavy nuclei split into lighter nuclei. B) Light nuclei form into heavier nuclei. C) Energy is released and less stable elements are formed. D) Energy is absorbed and more stable elements are formed. 30. When a nucleus with a high mass undergoes fission, the resulting nuclei are more stable than ...