An atom is an indivisible particle. is chemically indivisible. is the
... plutonium (atomic number 94). ...
... plutonium (atomic number 94). ...
power point notes
... If protons and neutrons have a mass of approx. 1 gram, then the total mass of the atom is equal to the total number of protons and neutrons Atomic mass = neutrons + protons Neutrons = atomic mass – atomic number ...
... If protons and neutrons have a mass of approx. 1 gram, then the total mass of the atom is equal to the total number of protons and neutrons Atomic mass = neutrons + protons Neutrons = atomic mass – atomic number ...
Atoms pg. 102
... According to the cloud model, electrons move rapidly in every direction depending on its energy level. ...
... According to the cloud model, electrons move rapidly in every direction depending on its energy level. ...
3b Atomic Theory Overview Unit 3b OVERVIEW atomic theory
... 11. When energy is added to an electron it will go from ground state to excited state. When it drops back to ground state energy is released in the form of light. 12. c = f 1) c is the symbol for the speed of light, the speed at which all electromagnetic radiation moves when in a perfect vacuum. (3 ...
... 11. When energy is added to an electron it will go from ground state to excited state. When it drops back to ground state energy is released in the form of light. 12. c = f 1) c is the symbol for the speed of light, the speed at which all electromagnetic radiation moves when in a perfect vacuum. (3 ...
Chemistry: The Nature of Matter
... Elements ____________________________________________________________ ____________________________________________________________ Periodic Table of Elements Over 100 elements known, but only about 2 dozen commonly found in living systems ...
... Elements ____________________________________________________________ ____________________________________________________________ Periodic Table of Elements Over 100 elements known, but only about 2 dozen commonly found in living systems ...
Atomic Structure
... He used a cathode ray tube to discover electrons. Sec. 1 - Atoms John Dalton and Democritus agreed that all elements are composed of _____________ particles called atoms. Unlike Democritus, Dalton performed ________________ to test and correct his theory. 100 000 000 copper atoms lined up would form ...
... He used a cathode ray tube to discover electrons. Sec. 1 - Atoms John Dalton and Democritus agreed that all elements are composed of _____________ particles called atoms. Unlike Democritus, Dalton performed ________________ to test and correct his theory. 100 000 000 copper atoms lined up would form ...
Worksheet - Chapter 3A - Atomic Structure 2012 Atomic Theory
... He used a cathode ray tube to discover electrons. Sec. 1 - Atoms John Dalton and Democritus agreed that all elements are composed of _____________ particles called atoms. Unlike Democritus, Dalton performed ________________ to test and correct his theory. 100 000 000 copper atoms lined up would form ...
... He used a cathode ray tube to discover electrons. Sec. 1 - Atoms John Dalton and Democritus agreed that all elements are composed of _____________ particles called atoms. Unlike Democritus, Dalton performed ________________ to test and correct his theory. 100 000 000 copper atoms lined up would form ...
Atom, Ion, Isotope Notes from 10/5 and 10/6
... a good estimation for finding the most common stable isotope of an atom. HOWEVER, it is not a perfect method. Look at Ag for example. It’s atomic mass is 107.87 amu, which would round to 108 amu. This is actually NOT a stable isotope of Ag (only 107 amu and 109 amu are). If you really wanted to know ...
... a good estimation for finding the most common stable isotope of an atom. HOWEVER, it is not a perfect method. Look at Ag for example. It’s atomic mass is 107.87 amu, which would round to 108 amu. This is actually NOT a stable isotope of Ag (only 107 amu and 109 amu are). If you really wanted to know ...
Atoms
... The nucleus of a typical atom has a radius of about 5 femtometers, or 0.000005 nanometers The red nucleus shown here is 100 times too large to be to scale to the yellow atom shown. ...
... The nucleus of a typical atom has a radius of about 5 femtometers, or 0.000005 nanometers The red nucleus shown here is 100 times too large to be to scale to the yellow atom shown. ...
SMP Quiz Session 1
... 1) The spli[ng of heavier nuclei to produce lighter nuclei and energy. 2) The combining of electrons with nuclei to produce atoms and release energy. 3) The process of fusing together light nuclei ...
... 1) The spli[ng of heavier nuclei to produce lighter nuclei and energy. 2) The combining of electrons with nuclei to produce atoms and release energy. 3) The process of fusing together light nuclei ...
AtomsIntro His
... number. • Mass numbers are found by adding the protons and neutrons. • Atomic mass of an element is the average mass of all the isotopes of that element. ...
... number. • Mass numbers are found by adding the protons and neutrons. • Atomic mass of an element is the average mass of all the isotopes of that element. ...
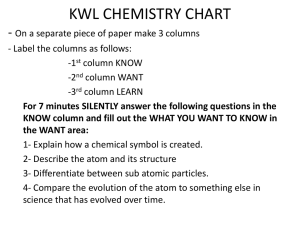
KWL chart and chem notes
... KNOW column and fill out the WHAT YOU WANT TO KNOW in the WANT area: 1- Explain how a chemical symbol is created. 2- Describe the atom and its structure 3- Differentiate between sub atomic particles. 4- Compare the evolution of the atom to something else in science that has evolved over time. ...
... KNOW column and fill out the WHAT YOU WANT TO KNOW in the WANT area: 1- Explain how a chemical symbol is created. 2- Describe the atom and its structure 3- Differentiate between sub atomic particles. 4- Compare the evolution of the atom to something else in science that has evolved over time. ...
How Atoms Differ Elements, Isotopes, and Ions
... Atoms are arranged in order by their atomic #. ...
... Atoms are arranged in order by their atomic #. ...
6.2 - Hockerill Students
... If you plot the neutron number N against the proton number Z for all the known nuclides, you get the diagram shown here Can you see that the stable nuclides of the lighter elements have approximately equal numbers of protons and neutrons? However, as Z increases the `stability line' curves upwards. ...
... If you plot the neutron number N against the proton number Z for all the known nuclides, you get the diagram shown here Can you see that the stable nuclides of the lighter elements have approximately equal numbers of protons and neutrons? However, as Z increases the `stability line' curves upwards. ...
Nuclear Fission and Nuclear Fusion
... Take a large atom and impact the nucleus with a particle. Split the atom releasing high energy, more high energy neutrons, and two daughter nuclides. Fission occurs only rarely in nature. Alpha decay is much more common. ...
... Take a large atom and impact the nucleus with a particle. Split the atom releasing high energy, more high energy neutrons, and two daughter nuclides. Fission occurs only rarely in nature. Alpha decay is much more common. ...
Identify which of the three subatomic particles (p+, n, e–): is the
... A) atomic number is the number that puts the elements in order and classifies how many electron and how many protons each element has ...
... A) atomic number is the number that puts the elements in order and classifies how many electron and how many protons each element has ...
Lecture 1: Basic Concepts: Atoms and Bonding
... an electron than others. Each electron does, however, have a specific energy. Must solve wave equation for specific states! • The combination of the energy and probability gives rise to the current understanding for electron distributions, which are referred to as electron orbitals; these orbital ...
... an electron than others. Each electron does, however, have a specific energy. Must solve wave equation for specific states! • The combination of the energy and probability gives rise to the current understanding for electron distributions, which are referred to as electron orbitals; these orbital ...
NUCLEAR CHEMISTRY
... immediately. Within a few days later the body breaks down very quickly since the gastrointestinal system is destroyed. Once the GI system ceases to function, nothing can be done, and medical care is for comfort only. 150 to 1,100 RAD: Severe blood changes will be noted and symptoms appear immediatel ...
... immediately. Within a few days later the body breaks down very quickly since the gastrointestinal system is destroyed. Once the GI system ceases to function, nothing can be done, and medical care is for comfort only. 150 to 1,100 RAD: Severe blood changes will be noted and symptoms appear immediatel ...
PreAP Chemistry Radioactivity WS Name Period ____ Match the
... ---------- 5. The weighted average mass of an element’s isotopes. ...
... ---------- 5. The weighted average mass of an element’s isotopes. ...
AtomicModelsandRadioactivity
... The joining of two small nuclei to form one larger one Again, a lot of energy is produced This is the process that powers the sun ...
... The joining of two small nuclei to form one larger one Again, a lot of energy is produced This is the process that powers the sun ...
Protons
... Over summer vacation, Debbie has to read a novel for English class. She has decided to spend the same amount of time reading every day. The number of hours she spends reading every day will determine how many days it will take her to finish the book. A small apple orchard hires fruit pickers to harv ...
... Over summer vacation, Debbie has to read a novel for English class. She has decided to spend the same amount of time reading every day. The number of hours she spends reading every day will determine how many days it will take her to finish the book. A small apple orchard hires fruit pickers to harv ...
Nucleus Electron Cloud Subatomic Particles
... Location: Outside of the Nucleus… mostly empty space Overall charge: Negative ...
... Location: Outside of the Nucleus… mostly empty space Overall charge: Negative ...
Linking Asteroids and Meteorites through Reflectance
... Atoms are made up of 3 types of particles Protons – positive charge (+1) Electrons – negative charge (-1) Neutrons – neutral charge (no charge) Protons and Neutrons are found in the nucleus ...
... Atoms are made up of 3 types of particles Protons – positive charge (+1) Electrons – negative charge (-1) Neutrons – neutral charge (no charge) Protons and Neutrons are found in the nucleus ...
Outline Chapter 8 The Nucleus 8-1. J.J. Thompson`s Plum Pudding
... radium. Radioactive decay occurs when a nucleus emits particles or high frequency em waves. ...
... radium. Radioactive decay occurs when a nucleus emits particles or high frequency em waves. ...
The Atomic Model - Mr. Brown`s Science Town
... positive dense core of the atom containing protons and neutrons Atom is mostly empty space where electrons randomly orbit nucleus. ...
... positive dense core of the atom containing protons and neutrons Atom is mostly empty space where electrons randomly orbit nucleus. ...