Atomic Structure AKS Correlation Use the modern atomic theory to
... Discovered e-, experiment, predictable shells, indivisible All matter made of plum pudding discovered p+, Bohr model 1st to think atoms model Atoms mostly about matter Matter cannot be empty space, dense and its’ makecreated or center up of atoms destroyed Atoms of same element look the same Chem re ...
... Discovered e-, experiment, predictable shells, indivisible All matter made of plum pudding discovered p+, Bohr model 1st to think atoms model Atoms mostly about matter Matter cannot be empty space, dense and its’ makecreated or center up of atoms destroyed Atoms of same element look the same Chem re ...
Honors Midterm - Stamford High School
... How can you separate a mixture? Paper chromatography can be used separate a mixture of inks, distillation can be used to separate a mixture of liquid, filtration can be used to separate a solid from a ...
... How can you separate a mixture? Paper chromatography can be used separate a mixture of inks, distillation can be used to separate a mixture of liquid, filtration can be used to separate a solid from a ...
Nuclear Radiation and Decay File
... elements, polonium and radium, that also were radioactive. • After more than three years, they were able to obtain about 0.1 g of radium from several tons of pitchblende (uranium oxide minerals). ...
... elements, polonium and radium, that also were radioactive. • After more than three years, they were able to obtain about 0.1 g of radium from several tons of pitchblende (uranium oxide minerals). ...
History of the Atom
... Basics on the atom: Three kinds of subatomic particles are electrons, protons, and neutrons. The nucleus is the center area of an atom and contains most of its mass Protons and neutrons are found in the nucleus. Each has a mass of 1 amu Electrons are found in levels outside of the nucleus ...
... Basics on the atom: Three kinds of subatomic particles are electrons, protons, and neutrons. The nucleus is the center area of an atom and contains most of its mass Protons and neutrons are found in the nucleus. Each has a mass of 1 amu Electrons are found in levels outside of the nucleus ...
NUCLEAR CHEMISTRY
... immediately. Within a few days later the body breaks down very quickly since the gastrointestinal system is destroyed. Once the GI system ceases to function, nothing can be done, and medical care is for comfort only. 150 to 1,100 RAD: Severe blood changes will be noted and symptoms appear immediatel ...
... immediately. Within a few days later the body breaks down very quickly since the gastrointestinal system is destroyed. Once the GI system ceases to function, nothing can be done, and medical care is for comfort only. 150 to 1,100 RAD: Severe blood changes will be noted and symptoms appear immediatel ...
The ATOM - Aarmstrongchem
... 1) All Matter is made up of very small particles called atoms 2) Atoms of the same element have the same chemical properties 3) While individual atoms of a given element may not all have the same mass any sample of the element will have a definite average mass that is characteristic. ...
... 1) All Matter is made up of very small particles called atoms 2) Atoms of the same element have the same chemical properties 3) While individual atoms of a given element may not all have the same mass any sample of the element will have a definite average mass that is characteristic. ...
Atoms - Science with Mrs. Schulte
... Atomic mass The average mass of all the isotopes (different types) of an element ...
... Atomic mass The average mass of all the isotopes (different types) of an element ...
8th-interlude-for-atoms - Epiphany Catholic School
... Compounds Formed when atoms of more than one element combined. Properties of a compound are very different from properties of the elements that make it up. Ex. NaCl = salt ...
... Compounds Formed when atoms of more than one element combined. Properties of a compound are very different from properties of the elements that make it up. Ex. NaCl = salt ...
Matter and the Periodic Table
... The Periodic Table In 1869 Dmitri Mendeleev (18341907) succeeded in organizing the 62 elements known at that time into a system of rows and columns on the basis of increasing mass and similar chemical and physical properties. Since the organization exhibited a periodic repetition of similar propert ...
... The Periodic Table In 1869 Dmitri Mendeleev (18341907) succeeded in organizing the 62 elements known at that time into a system of rows and columns on the basis of increasing mass and similar chemical and physical properties. Since the organization exhibited a periodic repetition of similar propert ...
Atomic Structure Worksheet Refer to your periodic table to fill in the
... • Too many electrons and the charge is negative. • Too few electrons and the charge is positive. EXAMPLE O-2 ...
... • Too many electrons and the charge is negative. • Too few electrons and the charge is positive. EXAMPLE O-2 ...
Speedy protons and the puzzling atomic nucleus
... which giant or pygmy resonances will form. The HECTOR and KRATTA systems of detectors are then be able to take detailed measurements of gamma quanta emitted by the vibrating nuclei, and thus to deduce their properties - such as the shape of the nucleus, distributions of neutrons relative to those of ...
... which giant or pygmy resonances will form. The HECTOR and KRATTA systems of detectors are then be able to take detailed measurements of gamma quanta emitted by the vibrating nuclei, and thus to deduce their properties - such as the shape of the nucleus, distributions of neutrons relative to those of ...
Nuclear Physics and Radioactivity
... atomic mass number (A) - the number of protons and neutrons in the nucleus of an atom. atomic mass unit - the unit of mass equal to 1/12 the mass of a carbon-12 nucleus; the atomic mass rounded to the nearest whole number is called the mass number. atomic number (Z) - the number of protons in the nu ...
... atomic mass number (A) - the number of protons and neutrons in the nucleus of an atom. atomic mass unit - the unit of mass equal to 1/12 the mass of a carbon-12 nucleus; the atomic mass rounded to the nearest whole number is called the mass number. atomic number (Z) - the number of protons in the nu ...
Atomic Structure and the Composition of Matter
... mass and are ~1800 times more massive than the electron. Both nuclear particles are composed of quarks, smaller fundamental particles. • Protons have unit positive charge (+1), while electrons have unit negative charge (-1). Neutrons ...
... mass and are ~1800 times more massive than the electron. Both nuclear particles are composed of quarks, smaller fundamental particles. • Protons have unit positive charge (+1), while electrons have unit negative charge (-1). Neutrons ...
Phys214 Final Exam
... 12. Consider two different samples of radioactive isotopes, one naturally occurring and the other artificially produced. If the samples have the same number of nuclei, then A. the one with the shorter half-life is likely more dangerous. B. the one with the smaller atomic mass is likely more dangerou ...
... 12. Consider two different samples of radioactive isotopes, one naturally occurring and the other artificially produced. If the samples have the same number of nuclei, then A. the one with the shorter half-life is likely more dangerous. B. the one with the smaller atomic mass is likely more dangerou ...
Atomic Structure - LFlemingPhysicalScience
... have additional electron clouds of different shapes that also show where those electrons are likely to be. For more information, click here: http://regentsprep.org/Regents/physics/phys05/catomodel/cloud.htm ...
... have additional electron clouds of different shapes that also show where those electrons are likely to be. For more information, click here: http://regentsprep.org/Regents/physics/phys05/catomodel/cloud.htm ...
History Atomic Theory
... – Know the name, location, charge, and relative mass of each of the subatomic particles in an atom – Know that the atomic number is the number of protons in the nucleus of an atom, and is unique to each element. – Understand that isotopes are atoms of the same element that differ in the number of ne ...
... – Know the name, location, charge, and relative mass of each of the subatomic particles in an atom – Know that the atomic number is the number of protons in the nucleus of an atom, and is unique to each element. – Understand that isotopes are atoms of the same element that differ in the number of ne ...
Objectives for Nuclear Chemistry
... There is natural and artificial radioactivity. Natural radioactivity is when a nucleus spontaneously decays into its product. In artificial radioactivity, the nucleus must be hit with a particle in order to make it unstable and cause it to emit radiation. The way to identify artificial vs. natural w ...
... There is natural and artificial radioactivity. Natural radioactivity is when a nucleus spontaneously decays into its product. In artificial radioactivity, the nucleus must be hit with a particle in order to make it unstable and cause it to emit radiation. The way to identify artificial vs. natural w ...
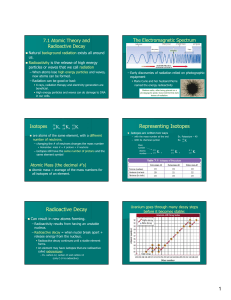
the # protons is not equal to the # neutrons
... Matter could be divided into smaller and smaller bits and still have the same properties. ...
... Matter could be divided into smaller and smaller bits and still have the same properties. ...
Models Atoms - Hardy Science
... 7. Atoms of the same element that have the same number of protons but different numbers of neutrons are called _________________. 8. The sum of protons and neutrons in the nucleus of an atom is the ____________________________________. 9. An electron’s movement is related to its ____________________ ...
... 7. Atoms of the same element that have the same number of protons but different numbers of neutrons are called _________________. 8. The sum of protons and neutrons in the nucleus of an atom is the ____________________________________. 9. An electron’s movement is related to its ____________________ ...
Big History Chemistry Study Guide File
... 4. Atoms are placed in the periodic table in order of _____________ __________________, which equals the number of __________________. The person who gets credit for this concept, and the first version of the table, is ______________ ___________________. 5. The atomic mass is equal to the __________ ...
... 4. Atoms are placed in the periodic table in order of _____________ __________________, which equals the number of __________________. The person who gets credit for this concept, and the first version of the table, is ______________ ___________________. 5. The atomic mass is equal to the __________ ...
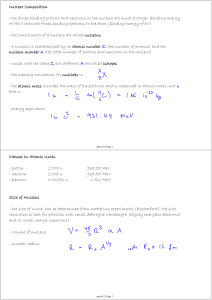
Masses in Atomic Units - proton 1.007 u 938.28 MeV
... - the binding energy of nucleons is extremely large compared to other atomic energy scales ...
... - the binding energy of nucleons is extremely large compared to other atomic energy scales ...