Accelerated Chemistry: Ch
... Nuclear chemistry is the study of the atomic nucleus and nuclear reactions. A nuclide is an atom consisting of 3 subatomic particles: electrons, protons and neutrons. A nucleon is the nucleus of an atom consisting of neutrons and protons. Keeping the Nucleus Together Two of the four known forces in ...
... Nuclear chemistry is the study of the atomic nucleus and nuclear reactions. A nuclide is an atom consisting of 3 subatomic particles: electrons, protons and neutrons. A nucleon is the nucleus of an atom consisting of neutrons and protons. Keeping the Nucleus Together Two of the four known forces in ...
A. The modern atomic model is based on the principles of . B. Greek
... J. Radioactive decay is caused by the ___________________________. K. The ______________________ is the center of the atom. L. Atoms of the same element that have the same number of protons but different number of neutrons are called _______________________. M. The subatomic particles that are n ...
... J. Radioactive decay is caused by the ___________________________. K. The ______________________ is the center of the atom. L. Atoms of the same element that have the same number of protons but different number of neutrons are called _______________________. M. The subatomic particles that are n ...
File
... identified by their mass, which is the total number of protons and neutrons. There are two ways that isotopes are generally written. They both use the mass of the atom where mass = (number of protons + number of neutrons). The first way is to put the mass as a superscript before the symbol of the el ...
... identified by their mass, which is the total number of protons and neutrons. There are two ways that isotopes are generally written. They both use the mass of the atom where mass = (number of protons + number of neutrons). The first way is to put the mass as a superscript before the symbol of the el ...
Name: Period: Atomic Theory Crossword Across 4. Who determined
... 12. Neutral subatomic particle. 13. Positively charged subatomic particle. 16. The earliest record of atomic theory was developed by this person. ...
... 12. Neutral subatomic particle. 13. Positively charged subatomic particle. 16. The earliest record of atomic theory was developed by this person. ...
Review 1st Qtr KEY
... CONCEPT QUESTIONS: Identify the letter of the choice that best completes the statement or answers the question. ANSWERS: A, B, B, B, B, B, C, C ____ 1. Most of the mass of an atom is found a. In the electron cloud. c. in the number of protons. b. in the nucleus. d. in the outer region of an atom. __ ...
... CONCEPT QUESTIONS: Identify the letter of the choice that best completes the statement or answers the question. ANSWERS: A, B, B, B, B, B, C, C ____ 1. Most of the mass of an atom is found a. In the electron cloud. c. in the number of protons. b. in the nucleus. d. in the outer region of an atom. __ ...
Radioactive Decay Series
... Artificial transmutation is the changing of the identity of a nucleus that is not occurring naturally Artificial transmutation happens by bombarding the nuclei with charged or uncharged particles Great quantities of energy is required to bombard the nuclei with these particles because of the repulsi ...
... Artificial transmutation is the changing of the identity of a nucleus that is not occurring naturally Artificial transmutation happens by bombarding the nuclei with charged or uncharged particles Great quantities of energy is required to bombard the nuclei with these particles because of the repulsi ...
A review of Atoms
... Note that the electron is found outside of the nucleus.Here it is shown as a small dot circling the nucleus. There are the same number of electrons and protons in a neutral atom. ...
... Note that the electron is found outside of the nucleus.Here it is shown as a small dot circling the nucleus. There are the same number of electrons and protons in a neutral atom. ...
Ch. 6 Vocabulary
... • atoms of the same element that have the same number of protons but a different number of neutrons ...
... • atoms of the same element that have the same number of protons but a different number of neutrons ...
Ch. 14.2 Notes
... 5. They are all carbon atoms because they all have six protons. These three kinds of carbon atoms are called _____________. 6. The isotopes of carbon are called _________________, ______________ and ________________. 7. The numbers tell _____________________________________________________________ _ ...
... 5. They are all carbon atoms because they all have six protons. These three kinds of carbon atoms are called _____________. 6. The isotopes of carbon are called _________________, ______________ and ________________. 7. The numbers tell _____________________________________________________________ _ ...
Atomic Structure/Electrons
... 11. His five postulates make up atomic theory. A 12. His gold foil experiment led to his discovery of the nucleus. C 13. He developed the planetary model of the atom, which described the light spectrum. D 14. What is the shape of a p orbital? a. sphere b. dumbbell c. rectangle d. flower 15. An atom ...
... 11. His five postulates make up atomic theory. A 12. His gold foil experiment led to his discovery of the nucleus. C 13. He developed the planetary model of the atom, which described the light spectrum. D 14. What is the shape of a p orbital? a. sphere b. dumbbell c. rectangle d. flower 15. An atom ...
Practice Clicker Questions: Atoms and Radioactivity, Introduction to
... Nucleons bound in a nucleus can have a different amount of energy (mass) than nucleons that are by themselves. Mass is a condensed form of energy! (E = mc2) ...
... Nucleons bound in a nucleus can have a different amount of energy (mass) than nucleons that are by themselves. Mass is a condensed form of energy! (E = mc2) ...
How to write up a practical: General review
... Objectives TO KNOW the terms protons, electrons and neutrons and some of their properties TO BE ABLE draw the basic structure of the atom. TO UNDERSTAND how these particles are physically arranged in relation to each other. ...
... Objectives TO KNOW the terms protons, electrons and neutrons and some of their properties TO BE ABLE draw the basic structure of the atom. TO UNDERSTAND how these particles are physically arranged in relation to each other. ...
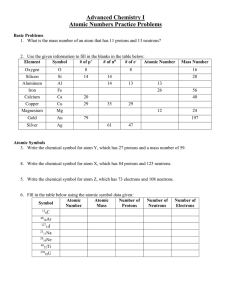
Atomic Numbers Practice Problems
... 3. Write the chemical symbol for atom Y, which has 27 protons and a mass number of 59. ...
... 3. Write the chemical symbol for atom Y, which has 27 protons and a mass number of 59. ...
Stoichiometry Introduction
... • Radioactivity: Is a natural process in which an unstable atom spontaneously transforms into a more stable atom, or several more stable atoms, while releasing energy in the form of radiation (p.126) ...
... • Radioactivity: Is a natural process in which an unstable atom spontaneously transforms into a more stable atom, or several more stable atoms, while releasing energy in the form of radiation (p.126) ...
Setting up Programmable PRS Keypad as Fixed ID Keypads
... Parser06 example6
The identity of an element is determined by the number of…
Q
Protons
Neutrons
Electrons
A molecule may consist of one atom.
Q
True
False
Molybdenum has atomic number 42. Its molar mass is 95.94 grams/mole. How many neutrons does
the most common isotope have?
Q
...
... Parser06 example
Atomic Structure Power Point
... So how can you have part of a neutron, such as 119.97 ? Because of ISOTOPES ! An isotope is a form of an element that has the same number of protons, but different numbers of neutrons. The atomic mass on the periodic table reflects the average mass of all of the known isotopes of an element. Each i ...
... So how can you have part of a neutron, such as 119.97 ? Because of ISOTOPES ! An isotope is a form of an element that has the same number of protons, but different numbers of neutrons. The atomic mass on the periodic table reflects the average mass of all of the known isotopes of an element. Each i ...
Physical Science Chapter 6 Study Guide Every element consists of
... o Different elements have different properties because their atoms are different o Atoms of different elements can combine in specific ways to form ____________ o Chemical processes are the result of the rearrangement, combination, or separation of atoms ...
... o Different elements have different properties because their atoms are different o Atoms of different elements can combine in specific ways to form ____________ o Chemical processes are the result of the rearrangement, combination, or separation of atoms ...
Section 1 Review
... 5. Infer Sodium and potassium are in the same group on the periodic table. Name ...
... 5. Infer Sodium and potassium are in the same group on the periodic table. Name ...
Modern Physics - hrsbstaff.ednet.ns.ca
... Isotopes of a given element correspond to nuclei with different numbers of neutrons. This results in a variety of different properties for the nuclei, including the obvious one of mass. The chemical behavior, however, is governed by the lectrons. All isotopes of a given element have the same number ...
... Isotopes of a given element correspond to nuclei with different numbers of neutrons. This results in a variety of different properties for the nuclei, including the obvious one of mass. The chemical behavior, however, is governed by the lectrons. All isotopes of a given element have the same number ...
Atomic Structure [PowerPoint]
... • The mass number is equal to the total number of protons plus the number of neutrons in the nucleus of an atom. • Atoms of the same element may have different number of neutrons, thus varying mass numbers. ...
... • The mass number is equal to the total number of protons plus the number of neutrons in the nucleus of an atom. • Atoms of the same element may have different number of neutrons, thus varying mass numbers. ...
Radioactivity - Miami Beach Senior High School
... Emitting beta particles • A neutron can spontaneously transform into a proton and electron in a nucleus with more neutrons than protons. • The electron is emitted form the nucleus. • This is beta radiation. • The element now increases its atomic number by one, as it has an extra proton. • Thorium-2 ...
... Emitting beta particles • A neutron can spontaneously transform into a proton and electron in a nucleus with more neutrons than protons. • The electron is emitted form the nucleus. • This is beta radiation. • The element now increases its atomic number by one, as it has an extra proton. • Thorium-2 ...
Physical Science Chapter 6 Study Guide Atomic Theory of Matter
... Parts of an atom o Nucleus—dense central core composed of protons and neutrons o Electrons surround the nucleus o Protons and neutrons are made up of small particles called quarks o Protons have positive charge and electrons have a negative charge ...
... Parts of an atom o Nucleus—dense central core composed of protons and neutrons o Electrons surround the nucleus o Protons and neutrons are made up of small particles called quarks o Protons have positive charge and electrons have a negative charge ...




















![Atomic Structure [PowerPoint]](http://s1.studyres.com/store/data/000122096_1-1d100da6540d2f26db122fc51f672fe5-300x300.png)