Lehninger Principles of Biochemistry 5/e
... produced by one round of the citric acid cycle? • CAC: 3 NADH, 1 FADH2, ...
... produced by one round of the citric acid cycle? • CAC: 3 NADH, 1 FADH2, ...
Cellular Respiration
... glycolysis, regenerating NAD+ needed for glycolysis to continue. •Lactic Acid Fermentation In lactic acid fermentation, bacteria and other animals covert pyruvate to lactic acid. Makes things SOUR! •Alcohol fermentation- Yeasts convert pyruvate to alcohol and CO2 ...
... glycolysis, regenerating NAD+ needed for glycolysis to continue. •Lactic Acid Fermentation In lactic acid fermentation, bacteria and other animals covert pyruvate to lactic acid. Makes things SOUR! •Alcohol fermentation- Yeasts convert pyruvate to alcohol and CO2 ...
Acyl-CoA
... - Triglycerides (or triacylglycerols) are fatty acid esters (usually with different fatty acid R groups) of glycerol—see §1.4! - Triglycerides are largely stored in the adipose tissue where they function as “high-energy” reservoirs—due to being more reduced (carry more electrons, or more hydrogens!) ...
... - Triglycerides (or triacylglycerols) are fatty acid esters (usually with different fatty acid R groups) of glycerol—see §1.4! - Triglycerides are largely stored in the adipose tissue where they function as “high-energy” reservoirs—due to being more reduced (carry more electrons, or more hydrogens!) ...
Chapter 10
... • Two electrons and one proton are transferred to NAD+ to form NADH • The bond between the a and b carbons is broken by a thiolase and a two-carbon fragment is transferred to a second acetyl CoA (FA-5) © 2012 Pearson Education, Inc. ...
... • Two electrons and one proton are transferred to NAD+ to form NADH • The bond between the a and b carbons is broken by a thiolase and a two-carbon fragment is transferred to a second acetyl CoA (FA-5) © 2012 Pearson Education, Inc. ...
Chapter 9: Pathways that Harvest Chemical
... This is an oxidation-reduction reaction. Glucose (C6H12O6) becomes completely oxidized and six molecules of O2 are reduced to six molecules of water. The energy that is released can be used to do work. The same equation applies to the overall metabolism of glucose in cells. However, in contrast to c ...
... This is an oxidation-reduction reaction. Glucose (C6H12O6) becomes completely oxidized and six molecules of O2 are reduced to six molecules of water. The energy that is released can be used to do work. The same equation applies to the overall metabolism of glucose in cells. However, in contrast to c ...
respiration 2010
... Respiration Take Place? • It actually takes place in two parts of the cell: Glycolysis occurs in the Cytoplasm ...
... Respiration Take Place? • It actually takes place in two parts of the cell: Glycolysis occurs in the Cytoplasm ...
Figure 4-24, step 1
... mitochondrial membrane High-energy electrons from glycolysis 1 Energy released 2 Energy from high-energy during metabolism electrons moving along is captured by highthe protein complexes energy electrons of the electron transport carried by NADH system pumps H+ from and FADH2. the matrix into the in ...
... mitochondrial membrane High-energy electrons from glycolysis 1 Energy released 2 Energy from high-energy during metabolism electrons moving along is captured by highthe protein complexes energy electrons of the electron transport carried by NADH system pumps H+ from and FADH2. the matrix into the in ...
Chapter 13 - TCA Cycle
... This oxidation is highly endergonic so [OAA] is always low. Exergonic reaction 1 pulls this forward. About –50 kJ /mol of G is released by the cycle and drives it in the direction of products. ...
... This oxidation is highly endergonic so [OAA] is always low. Exergonic reaction 1 pulls this forward. About –50 kJ /mol of G is released by the cycle and drives it in the direction of products. ...
Krebs cycle - Groby Bio Page
... 2 Idea that it is used to link reactions (1); idea that energy is released as a result of the activity of one enzyme and used by another enzyme (1). ...
... 2 Idea that it is used to link reactions (1); idea that energy is released as a result of the activity of one enzyme and used by another enzyme (1). ...
... FADH is oxidized in complex II – technically it shuttles electrons from its substrate to FeS centers, electrons go on Q to form QH2 (4 pts). Protons are pumped across the membrane in complex I, but not complex II (2 pts) The rest of the pathway is the same QH2 to Complex III to cytochrome C to compl ...
Citric Acid Cycle
... minerals. Cofactor can be an organic molecule, metal ion, or organometallic complex. Cofactors can be either: • Cosubstrate = small organic molecule that associates only transiently with an enzyme. Later associates with another enzyme. • Prosthetic Group = a molecule that is permanently and tightly ...
... minerals. Cofactor can be an organic molecule, metal ion, or organometallic complex. Cofactors can be either: • Cosubstrate = small organic molecule that associates only transiently with an enzyme. Later associates with another enzyme. • Prosthetic Group = a molecule that is permanently and tightly ...
Redox cycling
... The Gibbs free energy is related to the redox potential: G = - nF E (E is the difference in redox potential between two reacting compounds) Elias Arnér ...
... The Gibbs free energy is related to the redox potential: G = - nF E (E is the difference in redox potential between two reacting compounds) Elias Arnér ...
AP Review
... Acetyl CoA will enter the Krebs cycle for further oxidation Krebs cycle - Acetyl CoA (2C) enters, 2 CO2 (1C) leave, 3 NAD+ 3 NADH, 1 FAD 1 FADH2, 1 ADP 1 ATP ...
... Acetyl CoA will enter the Krebs cycle for further oxidation Krebs cycle - Acetyl CoA (2C) enters, 2 CO2 (1C) leave, 3 NAD+ 3 NADH, 1 FAD 1 FADH2, 1 ADP 1 ATP ...
Photosynthesis
... Autotrophs are the producers of the biosphere, producing organic molecules from CO2 and other inorganic molecules Almost all plants are photoautotrophs, using the energy of sunlight to make organic molecules from water and carbon dioxide ...
... Autotrophs are the producers of the biosphere, producing organic molecules from CO2 and other inorganic molecules Almost all plants are photoautotrophs, using the energy of sunlight to make organic molecules from water and carbon dioxide ...
Cellular respiration
... enter the mitochondrion and are completely broken down, yielding two ATP and ten high-energy electron carriers: eight NADH and two FADH2. The carbon atoms from the pyruvates are released in six molecules of CO2 – High-energy electrons release energy that is harnessed to pump H into the intermembran ...
... enter the mitochondrion and are completely broken down, yielding two ATP and ten high-energy electron carriers: eight NADH and two FADH2. The carbon atoms from the pyruvates are released in six molecules of CO2 – High-energy electrons release energy that is harnessed to pump H into the intermembran ...
LIPIDS - Biochemistry Notes
... metabolized for energy; in diabetes, the glucose is not available for glucolysis due to the shortage of insulin that prevents the glucose entry in the cell; thus, acetyl-CoA is used preferentially over glucose as an energy ...
... metabolized for energy; in diabetes, the glucose is not available for glucolysis due to the shortage of insulin that prevents the glucose entry in the cell; thus, acetyl-CoA is used preferentially over glucose as an energy ...
Lecture 2 - Washington State University
... Copyright © The McGraw-Hill Companies, Inc. Permission required for reproduction or display. ...
... Copyright © The McGraw-Hill Companies, Inc. Permission required for reproduction or display. ...
Internal expression of Yarrowia NDH2
... 2000b) in the diploid strain GB1, we failed to isolate haploid spores carrying these deletion alleles, either by random spore analysis or by ascus dissection. As we also failed to introduce the deletion alleles directly into haploid yeast strains, it seemed that complex I was essential for vegetativ ...
... 2000b) in the diploid strain GB1, we failed to isolate haploid spores carrying these deletion alleles, either by random spore analysis or by ascus dissection. As we also failed to introduce the deletion alleles directly into haploid yeast strains, it seemed that complex I was essential for vegetativ ...
Carbohydrate
... series of enzyme-catalyzed reactions to yield two molecules of the three-carbon compound pyruvate . During the sequential reactions of glycolysis, some of the free energy released from glucose is conserved in the form of ATP and NADH. ...
... series of enzyme-catalyzed reactions to yield two molecules of the three-carbon compound pyruvate . During the sequential reactions of glycolysis, some of the free energy released from glucose is conserved in the form of ATP and NADH. ...
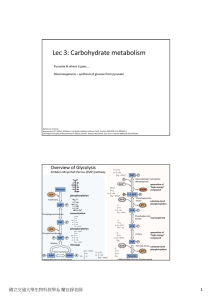
Lec 3: Carbohydrate metabolism
... Pyruvate can return upstream central metabolism through gluconeogenesis. Intermediates of glycolysis/ gluconeogenesis are precursors to many amino acids, nucleotides, etc. ...
... Pyruvate can return upstream central metabolism through gluconeogenesis. Intermediates of glycolysis/ gluconeogenesis are precursors to many amino acids, nucleotides, etc. ...
Electron transport chain
An electron transport chain (ETC) is a series of compounds that transfer electrons from electron donors to electron acceptors via redox reactions, and couples this electron transfer with the transfer of protons (H+ ions) across a membrane. This creates an electrochemical proton gradient that drives ATP synthesis, or the generation of chemical energy in the form of adenosine triphosphate (ATP). The final acceptor of electrons in the electron transport chain is molecular oxygen.Electron transport chains are used for extracting energy via redox reactions from sunlight in photosynthesis or, such as in the case of the oxidation of sugars, cellular respiration. In eukaryotes, an important electron transport chain is found in the inner mitochondrial membrane where it serves as the site of oxidative phosphorylation through the use of ATP synthase. It is also found in the thylakoid membrane of the chloroplast in photosynthetic eukaryotes. In bacteria, the electron transport chain is located in their cell membrane.In chloroplasts, light drives the conversion of water to oxygen and NADP+ to NADPH with transfer of H+ ions across chloroplast membranes. In mitochondria, it is the conversion of oxygen to water, NADH to NAD+ and succinate to fumarate that are required to generate the proton gradient. Electron transport chains are major sites of premature electron leakage to oxygen, generating superoxide and potentially resulting in increased oxidative stress.