Bell work
... and Krebs to create ATP • Electron Transport - NAPH and FADH pass from carrier to carrier to power hydrogen pumps and create a hydrogen gradient in inter-membrane space! • ATP Production ATP synthase allows H to flow through like turbine (into matix) and produce ATP ...
... and Krebs to create ATP • Electron Transport - NAPH and FADH pass from carrier to carrier to power hydrogen pumps and create a hydrogen gradient in inter-membrane space! • ATP Production ATP synthase allows H to flow through like turbine (into matix) and produce ATP ...
Summary
... Aerobic “respiration” in mitochondria: important for ATP synthesis and a source of intermediates for other biochemical pathways pyruvate (C3H3O3) + 4 NAD+ + FAD + GDP + Pi + 2H2O ...
... Aerobic “respiration” in mitochondria: important for ATP synthesis and a source of intermediates for other biochemical pathways pyruvate (C3H3O3) + 4 NAD+ + FAD + GDP + Pi + 2H2O ...
... Choice A: The energy released by degradative pathways is directly captured on which types of compounds? Briefly explain how the energy on these compounds is converted to a hydrogen ion (proton) gradient across a membrane during electron transport. Choice B: Briefly explain how the hydrogen ion gradi ...
Name
... KEY CONCEPT Fermentation allows the production of a small amount of ATP without oxygen. When oxygen is not available in cells, fermentation takes place instead. Fermentation is an anaerobic process that allows glycolysis to continue, but does not produce ATP on its own. The main function of fermenta ...
... KEY CONCEPT Fermentation allows the production of a small amount of ATP without oxygen. When oxygen is not available in cells, fermentation takes place instead. Fermentation is an anaerobic process that allows glycolysis to continue, but does not produce ATP on its own. The main function of fermenta ...
Ch - wlhs.wlwv.k12.or.us
... Regulation of Cellular Respiration via Feedback Mechanisms ● FEEDBACK INHIBITION is the most common mechanism for control ● If ATP concentration begins to drop, ● when there is plenty of ATP, ● Control of catabolism is based mainly on regulating the ...
... Regulation of Cellular Respiration via Feedback Mechanisms ● FEEDBACK INHIBITION is the most common mechanism for control ● If ATP concentration begins to drop, ● when there is plenty of ATP, ● Control of catabolism is based mainly on regulating the ...
Metabolic Engineering for Fuels and Chemicals
... (Mixed acid, ethanol, lactate, acetate, pyruvate, glutamate, succinate, alanine, citrate) ¾ATP/ADP? ...
... (Mixed acid, ethanol, lactate, acetate, pyruvate, glutamate, succinate, alanine, citrate) ¾ATP/ADP? ...
Document
... malate-aspartate shuttle is used. Electrons (H+) from NADH are used to generate malate (from oxaloacetate), which crosses the membrane and gives them back to NAD to generate NADH (and oxaloacetate). Oxaloacetate is converted into aspartate, which crosses back to the cytosol and is metabolized to oxa ...
... malate-aspartate shuttle is used. Electrons (H+) from NADH are used to generate malate (from oxaloacetate), which crosses the membrane and gives them back to NAD to generate NADH (and oxaloacetate). Oxaloacetate is converted into aspartate, which crosses back to the cytosol and is metabolized to oxa ...
IOSR Journal of Pharmacy and Biological Sciences (IOSR-JPBS)
... Mitochondrial aging is characterized by destruction of structural integrity of the membrane, leading to a decline in mitochondrial membrane fluidity and activities of enzymes associated with membrane lipids [1]. As the activities of most enzymes are regulated by the physicochemical state of the lipi ...
... Mitochondrial aging is characterized by destruction of structural integrity of the membrane, leading to a decline in mitochondrial membrane fluidity and activities of enzymes associated with membrane lipids [1]. As the activities of most enzymes are regulated by the physicochemical state of the lipi ...
Mitochondrial permeability transition pore
... (adenine nucleotide translocator) CypD (cyclophilin D) exhibits peptidyl-prolyl cis-trans isomerase(PIPase) activity. ...
... (adenine nucleotide translocator) CypD (cyclophilin D) exhibits peptidyl-prolyl cis-trans isomerase(PIPase) activity. ...
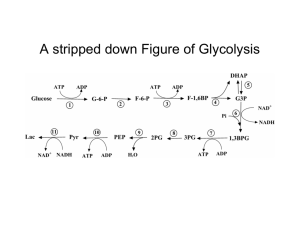
Figure 17-3 Degradation of glucose via the glycolytic pathway.
... by NADH. Thus, no net oxidation occurs in glycolysis = fermentation; another organic serving as electron acceptor. •lactate, end-product under anaerobic conditions, diffuses thru cell membrane as waste into blood - salvaged by liver and rebuilt to form glucose (gluconeogenesis). This occurs in skele ...
... by NADH. Thus, no net oxidation occurs in glycolysis = fermentation; another organic serving as electron acceptor. •lactate, end-product under anaerobic conditions, diffuses thru cell membrane as waste into blood - salvaged by liver and rebuilt to form glucose (gluconeogenesis). This occurs in skele ...
Cellular Energy and Mitochondrial ATP Production: A
... mitochondrial membrane. And by means of a very complicated series of events the electron carriers NADH and FADH2 - produced during the earlier stages of glycolysis, pyruvate oxidation, Krebs cycle - are now used to create a high gradient of hydrogen atoms in the outer mitochondrial compartment. This ...
... mitochondrial membrane. And by means of a very complicated series of events the electron carriers NADH and FADH2 - produced during the earlier stages of glycolysis, pyruvate oxidation, Krebs cycle - are now used to create a high gradient of hydrogen atoms in the outer mitochondrial compartment. This ...
CHEMISTRY OF FOOD FERMENTATION
... Fermentation is a form of anaerobic digestion that generates adenosine triphosphate (ATP) by the process of substrate-level phosphorylation. The energy for generating ATP comes from the oxidation of organic compounds, such as carbohydrates. In contrast, during respiration the energy for ATP formatio ...
... Fermentation is a form of anaerobic digestion that generates adenosine triphosphate (ATP) by the process of substrate-level phosphorylation. The energy for generating ATP comes from the oxidation of organic compounds, such as carbohydrates. In contrast, during respiration the energy for ATP formatio ...
Name Date ______ Your
... F. Define Anaerobic Process: ________________________________________________________ G. Define Aerobic Respiration: ______________________________________________________ ...
... F. Define Anaerobic Process: ________________________________________________________ G. Define Aerobic Respiration: ______________________________________________________ ...
Succinate Dehydrogenase of Saccharomyces cerevisiae
... Two electrons from the reduced SDH-FADH2 complex are then transferred to ubiquinone (Q), a soluble component of the electron transport system complex II. Ubiquinone is then reduced to ubiquinol ( QH2). Flavin adenine dinucleotide (FAD) is an essential cofactor for SDH enzyme. The generation of adeno ...
... Two electrons from the reduced SDH-FADH2 complex are then transferred to ubiquinone (Q), a soluble component of the electron transport system complex II. Ubiquinone is then reduced to ubiquinol ( QH2). Flavin adenine dinucleotide (FAD) is an essential cofactor for SDH enzyme. The generation of adeno ...
Slide 1
... I. Oxidation & Reduction -a substance which ________ oxidizes another substance by ________ accepting its ________ electrons is called an ________ oxidizing _____, agent which is also reduced the substance that is _______ -a substance which _______ reduces another substance by ______ losing ________ ...
... I. Oxidation & Reduction -a substance which ________ oxidizes another substance by ________ accepting its ________ electrons is called an ________ oxidizing _____, agent which is also reduced the substance that is _______ -a substance which _______ reduces another substance by ______ losing ________ ...
GLYCOLYSIS (1).
... • It occurs in the cytosol of all cells. • Its unique features is that it can function aerobically or anaerobically, depending on the availability of oxygen and electron transport chain. • RBCs have no mitochondria and they rely completely on glucose as their metabolic fuel and metabolize it anaerob ...
... • It occurs in the cytosol of all cells. • Its unique features is that it can function aerobically or anaerobically, depending on the availability of oxygen and electron transport chain. • RBCs have no mitochondria and they rely completely on glucose as their metabolic fuel and metabolize it anaerob ...
GLYCOLYSIS
... • It occurs in the cytosol of all cells. • Its unique features is that it can function aerobically or anaerobically, depending on the availability of oxygen and electron transport chain. • RBCs have no mitochondria and they rely completely on glucose as their metabolic fuel and metabolize it anaerob ...
... • It occurs in the cytosol of all cells. • Its unique features is that it can function aerobically or anaerobically, depending on the availability of oxygen and electron transport chain. • RBCs have no mitochondria and they rely completely on glucose as their metabolic fuel and metabolize it anaerob ...
Ecological speciation model
... Pyruvate decarboxylase Substrate-level phosphorylation: PEP + ADP -> pyruvate + ATP 2 CO2 1,3-bisphosphoglycerate + ADP -> 3 phosphoglycerate + ATP 2 acetaldehyde H3C C O H Net ATP 2 NADH use 2 ATP make 4 ATP ...
... Pyruvate decarboxylase Substrate-level phosphorylation: PEP + ADP -> pyruvate + ATP 2 CO2 1,3-bisphosphoglycerate + ADP -> 3 phosphoglycerate + ATP 2 acetaldehyde H3C C O H Net ATP 2 NADH use 2 ATP make 4 ATP ...
Glycolysis - medscistudents
... Fluoride irreversibly inhibit this enzyme with the removal of Mg2+ High energy of Phosphoenolpyruvate is trapped into ATP by the pyruvate kinase, irreversible reaction. Ends with pyruvate in the tissues with mitochondria (aerobic) If anaerobic conditions prevail, the reoxidation of NADH formed in re ...
... Fluoride irreversibly inhibit this enzyme with the removal of Mg2+ High energy of Phosphoenolpyruvate is trapped into ATP by the pyruvate kinase, irreversible reaction. Ends with pyruvate in the tissues with mitochondria (aerobic) If anaerobic conditions prevail, the reoxidation of NADH formed in re ...
Electron transport chain
An electron transport chain (ETC) is a series of compounds that transfer electrons from electron donors to electron acceptors via redox reactions, and couples this electron transfer with the transfer of protons (H+ ions) across a membrane. This creates an electrochemical proton gradient that drives ATP synthesis, or the generation of chemical energy in the form of adenosine triphosphate (ATP). The final acceptor of electrons in the electron transport chain is molecular oxygen.Electron transport chains are used for extracting energy via redox reactions from sunlight in photosynthesis or, such as in the case of the oxidation of sugars, cellular respiration. In eukaryotes, an important electron transport chain is found in the inner mitochondrial membrane where it serves as the site of oxidative phosphorylation through the use of ATP synthase. It is also found in the thylakoid membrane of the chloroplast in photosynthetic eukaryotes. In bacteria, the electron transport chain is located in their cell membrane.In chloroplasts, light drives the conversion of water to oxygen and NADP+ to NADPH with transfer of H+ ions across chloroplast membranes. In mitochondria, it is the conversion of oxygen to water, NADH to NAD+ and succinate to fumarate that are required to generate the proton gradient. Electron transport chains are major sites of premature electron leakage to oxygen, generating superoxide and potentially resulting in increased oxidative stress.