Democritus John Dalton Joseph John (J.J.) Thomson Dimitri
... First to develop idea that matter was made of particles called “atoms”- from the Greek word “atomos”, which means “indivisible”. ...
... First to develop idea that matter was made of particles called “atoms”- from the Greek word “atomos”, which means “indivisible”. ...
Earth`s Chemistry
... All matter is made up of elements. Element = any substance that can’t break down any further (Ex. oxygen, iron, nitrogen, etc.). Periodic table --- made up of elements. Universal symbol for elements --- consists of either one letter or two (Note how it’s written) Over 90 elements occur naturally; ot ...
... All matter is made up of elements. Element = any substance that can’t break down any further (Ex. oxygen, iron, nitrogen, etc.). Periodic table --- made up of elements. Universal symbol for elements --- consists of either one letter or two (Note how it’s written) Over 90 elements occur naturally; ot ...
Ch. 18 Notes Atoms and Elements
... electrons occupy 8eThe electron cloud is Up to 18esubdivided into smaller ...
... electrons occupy 8eThe electron cloud is Up to 18esubdivided into smaller ...
Chem 101A Exam 4 Concepts Chapter 7 – Modern Atomic Theory
... Electron configurations of a given atom 1s22s22p6…etc Short/abbreviated method, i.e. [noble gas]…the rest Orbital diagrams (and Hund’s Rule) Exceptions to predicted electron configs (Cu,Ag,Au) Valence electrons Periodic table and electron configs (e.g. alkaline earth metals last ...
... Electron configurations of a given atom 1s22s22p6…etc Short/abbreviated method, i.e. [noble gas]…the rest Orbital diagrams (and Hund’s Rule) Exceptions to predicted electron configs (Cu,Ag,Au) Valence electrons Periodic table and electron configs (e.g. alkaline earth metals last ...
Atoms and Elements
... Electrons circle the nucleus in a paths called orbits or energy levels. Low-energy = orbit close to nucleus High-energy = orbit father away. Most of an atoms mass is in the nucleus; protons and neutrons have the same mass; electrons as about 1/2000 of a proton ...
... Electrons circle the nucleus in a paths called orbits or energy levels. Low-energy = orbit close to nucleus High-energy = orbit father away. Most of an atoms mass is in the nucleus; protons and neutrons have the same mass; electrons as about 1/2000 of a proton ...
What does the Periodic Table tell us?
... Any element with an atomic number greater than ____ is man-made (created in a lab) Why is there usually a decimal place in the atomic mass number? It is due to _________________________ Isotopes – atoms of the same element with the same # of ____________ but a different # of _____________ th ...
... Any element with an atomic number greater than ____ is man-made (created in a lab) Why is there usually a decimal place in the atomic mass number? It is due to _________________________ Isotopes – atoms of the same element with the same # of ____________ but a different # of _____________ th ...
Document
... a) an element which has 5 electrons in each atom b) an element which has 5 electrons in its outer energy level c) an element for which the second energy level is completely filled d) an element which forms ions by gaining only one electron e) how many elements are there in the sixth period? f) the e ...
... a) an element which has 5 electrons in each atom b) an element which has 5 electrons in its outer energy level c) an element for which the second energy level is completely filled d) an element which forms ions by gaining only one electron e) how many elements are there in the sixth period? f) the e ...
GY 111 Lecture Note Series Elemental Chemistry
... various numbers of these particles. Protons always carry a single positive charge; electrons always carry a single negative charge and neutrons are as the name implies, neutral or non charged. Each atom contains an equal number of protons and electrons; ions contain differing numbers. For example: ...
... various numbers of these particles. Protons always carry a single positive charge; electrons always carry a single negative charge and neutrons are as the name implies, neutral or non charged. Each atom contains an equal number of protons and electrons; ions contain differing numbers. For example: ...
SCH3U Course Review
... 10. Explain and illustrate the variation of the following in the Periodic Table: (a) ionization energy (b) electron affinity (c) atomic radius 11. For the following elements: (a) state if they tend to gain or lose electrons (b) how many electrons are gained or lost. (a) H (b) Na (c) Cl (d) Mg (e) S ...
... 10. Explain and illustrate the variation of the following in the Periodic Table: (a) ionization energy (b) electron affinity (c) atomic radius 11. For the following elements: (a) state if they tend to gain or lose electrons (b) how many electrons are gained or lost. (a) H (b) Na (c) Cl (d) Mg (e) S ...
Physical and Chemical Properties
... • Arranged by increasing atomic number (proton #) • Rows are called periods & are labeled 1-7 • There are 18 columns • Each tall column contains a group or family of elements. • Groups are elements that have similar physical or chemical properties. • Ex. All elements in group 1 are metals & react vi ...
... • Arranged by increasing atomic number (proton #) • Rows are called periods & are labeled 1-7 • There are 18 columns • Each tall column contains a group or family of elements. • Groups are elements that have similar physical or chemical properties. • Ex. All elements in group 1 are metals & react vi ...
File
... 20. Is sodium a metal or non-metal and list at least 3 properties of sodium? Metal; highly reactive, metal, one valence electron 21. List two Noble Gases and explain why they are unique. Neon and Argon have full valence electron levels making them very stable 22. List two Alkali Metals and explain w ...
... 20. Is sodium a metal or non-metal and list at least 3 properties of sodium? Metal; highly reactive, metal, one valence electron 21. List two Noble Gases and explain why they are unique. Neon and Argon have full valence electron levels making them very stable 22. List two Alkali Metals and explain w ...
Chapter 1: Chemistry and You
... are good representation of what to expect on the midterm, but it is not enough to just study from the review. You need to look over your notes, old review sheets, tests and quizzes, homework, etc. Ask questions!!!! You may check your answers online and I am available Tuesday, Wednesday, and Thursday ...
... are good representation of what to expect on the midterm, but it is not enough to just study from the review. You need to look over your notes, old review sheets, tests and quizzes, homework, etc. Ask questions!!!! You may check your answers online and I am available Tuesday, Wednesday, and Thursday ...
key
... Placed in table above using blue electrons. We predict it to be a colorless gas with low electrical conductivity and high electrical reactivity. c) Are there any elements that have not yet been discovered? If so, what would their properties be? This table has room for four more elements. The element ...
... Placed in table above using blue electrons. We predict it to be a colorless gas with low electrical conductivity and high electrical reactivity. c) Are there any elements that have not yet been discovered? If so, what would their properties be? This table has room for four more elements. The element ...
Name - Aurora City Schools
... 9. __ __ any charged particle, an atom that has gained or lost electrons ...
... 9. __ __ any charged particle, an atom that has gained or lost electrons ...
Name - Aurora City Schools
... 9. __ __ any charged particle, an atom that has gained or lost electrons ...
... 9. __ __ any charged particle, an atom that has gained or lost electrons ...
ATOMS and PERIODIC TABLE - John Q. Adams Middle School
... of the same element do not always have the same number of neutrons. Atoms that have different numbers of neutrons are called isotopes. The atomic mass given on the periodic table is the average of all the isotopes and is not a whole number. To find the number of neutrons for an element, subtra ...
... of the same element do not always have the same number of neutrons. Atoms that have different numbers of neutrons are called isotopes. The atomic mass given on the periodic table is the average of all the isotopes and is not a whole number. To find the number of neutrons for an element, subtra ...
Basic Chemistry Lesson - Agriculture Solutions
... distinguish between elements, each element is assigned an atomic number (we rarely refer to these numbers during the course). The atomic number is the number of protons in the nucleus of an atom of that element. You will see on the supplied Periodic Table, the atomic number for hydrogen (H) is 1, wh ...
... distinguish between elements, each element is assigned an atomic number (we rarely refer to these numbers during the course). The atomic number is the number of protons in the nucleus of an atom of that element. You will see on the supplied Periodic Table, the atomic number for hydrogen (H) is 1, wh ...
CHE 1401 - Fall 2013 - Chapter 7 Homework 7 (Chapter 7: Periodic
... 11) Hydrogen is unique among the elements because __________. 1. It is not really a member of any particular group. 2. Its electron is not at all shielded from its nucleus. 3. It is the lightest element. 4. It is the only element to exist at room temperature as a diatomic gas. 5. It exhibits some c ...
... 11) Hydrogen is unique among the elements because __________. 1. It is not really a member of any particular group. 2. Its electron is not at all shielded from its nucleus. 3. It is the lightest element. 4. It is the only element to exist at room temperature as a diatomic gas. 5. It exhibits some c ...
Chapter 3 Atoms and Elements
... Also on closer inspection of the different n levels, additional fine structure is observed within each n level and these are assigned different letters of the alphabet. According to the mathematics each s, p, d level can accommodate 2 electrons. There is one s level for each shelf, three equivalent ...
... Also on closer inspection of the different n levels, additional fine structure is observed within each n level and these are assigned different letters of the alphabet. According to the mathematics each s, p, d level can accommodate 2 electrons. There is one s level for each shelf, three equivalent ...
Notes - Organization of Matter
... Periodic Table of Elements (PTE) • Used to organize all of the elements in a visual way • Every periodic table will have a square for each element with the: • element name • chemical/element symbol • atomic number • atomic mass ...
... Periodic Table of Elements (PTE) • Used to organize all of the elements in a visual way • Every periodic table will have a square for each element with the: • element name • chemical/element symbol • atomic number • atomic mass ...
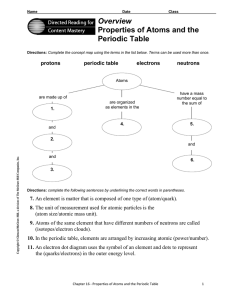
Overview Properties of Atoms and the Periodic Table
... than will other atoms of the same element. Atoms of the same ...
... than will other atoms of the same element. Atoms of the same ...
There are 3 particles in an atom
... Protons have a positive charge and are found in the nucleus (center) of an atom. They are always equal to the atomic number on the periodic table (top number in the box). Neutrons have no charge. They are neutral. They are also found in the center of an atom. Electrons have a negative charge. They a ...
... Protons have a positive charge and are found in the nucleus (center) of an atom. They are always equal to the atomic number on the periodic table (top number in the box). Neutrons have no charge. They are neutral. They are also found in the center of an atom. Electrons have a negative charge. They a ...
Periodic table
The periodic table is a tabular arrangement of the chemical elements, ordered by their atomic number (number of protons in the nucleus), electron configurations, and recurring chemical properties. The table also shows four rectangular blocks: s-, p- d- and f-block. In general, within one row (period) the elements are metals on the lefthand side, and non-metals on the righthand side.The rows of the table are called periods; the columns are called groups. Six groups (columns) have names as well as numbers: for example, group 17 elements are the halogens; and group 18, the noble gases. The periodic table can be used to derive relationships between the properties of the elements, and predict the properties of new elements yet to be discovered or synthesized. The periodic table provides a useful framework for analyzing chemical behavior, and is widely used in chemistry and other sciences.Although precursors exist, Dmitri Mendeleev is generally credited with the publication, in 1869, of the first widely recognized periodic table. He developed his table to illustrate periodic trends in the properties of the then-known elements. Mendeleev also predicted some properties of then-unknown elements that would be expected to fill gaps in this table. Most of his predictions were proved correct when the elements in question were subsequently discovered. Mendeleev's periodic table has since been expanded and refined with the discovery or synthesis of further new elements and the development of new theoretical models to explain chemical behavior.All elements from atomic numbers 1 (hydrogen) to 118 (ununoctium) have been discovered or reportedly synthesized, with elements 113, 115, 117, and 118 having yet to be confirmed. The first 94 elements exist naturally, although some are found only in trace amounts and were synthesized in laboratories before being found in nature. Elements with atomic numbers from 95 to 118 have only been synthesized in laboratories. It has been shown that einsteinium and fermium once occurred in nature but currently do not. Synthesis of elements having higher atomic numbers is being pursued. Numerous synthetic radionuclides of naturally occurring elements have also been produced in laboratories.