Review for Periodic - Mr-Durands
... 3. What is the number of neutrons for Cesium (Cs)? 4. What is the difference between atomic mass and number? 5. How do isotopes affect the atomic mass of an element? 6. What is a group on the periodic table? 7. How many groups are there? 8. What is a period on the periodic table? 9. How many periods ...
... 3. What is the number of neutrons for Cesium (Cs)? 4. What is the difference between atomic mass and number? 5. How do isotopes affect the atomic mass of an element? 6. What is a group on the periodic table? 7. How many groups are there? 8. What is a period on the periodic table? 9. How many periods ...
Periodic Table
... –Included elements Valence # (bonding power) •How many e- the atom can lose or gain when bonding •Pattern appeared - 1234321 ...
... –Included elements Valence # (bonding power) •How many e- the atom can lose or gain when bonding •Pattern appeared - 1234321 ...
Periodic Table Puzzle
... The code letters A to Z have been assigned to represent the first 26 representative elements in the Periodic Table. The letters do not relate to the actual chemical symbols for these elements. Your challenge is to put the code letters in the correct boxes in the Periodic Table, based on the properti ...
... The code letters A to Z have been assigned to represent the first 26 representative elements in the Periodic Table. The letters do not relate to the actual chemical symbols for these elements. Your challenge is to put the code letters in the correct boxes in the Periodic Table, based on the properti ...
Chapter 5
... •Designed by a group of scientists. •Electron cloud is 10,000 times larger than the nucleus, but is still mostly empty. •Electrons are in the cloud but can not be pinpointed at an exact time because they move so quickly. ...
... •Designed by a group of scientists. •Electron cloud is 10,000 times larger than the nucleus, but is still mostly empty. •Electrons are in the cloud but can not be pinpointed at an exact time because they move so quickly. ...
2.2 Periodic Trends
... What are the trends that occur in the periodic table by organizing elements by their atomic number? Periodic trends are specific patterns that are present in the periodic table that illustrate different aspects of a certain element. Periodic trends, arising from the arrangement of the periodic t ...
... What are the trends that occur in the periodic table by organizing elements by their atomic number? Periodic trends are specific patterns that are present in the periodic table that illustrate different aspects of a certain element. Periodic trends, arising from the arrangement of the periodic t ...
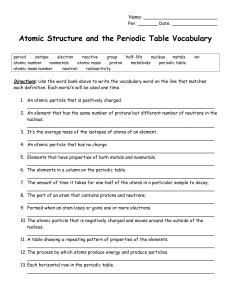
Atomic Structure and the Periodic Table Vocabulary
... 2. An element that has the same number of protons but different number of neutrons in the nucleus. __________________________________________________________________ 3. It’s the average mass of the isotopes of atoms of an element. __________________________________________________________________ 4. ...
... 2. An element that has the same number of protons but different number of neutrons in the nucleus. __________________________________________________________________ 3. It’s the average mass of the isotopes of atoms of an element. __________________________________________________________________ 4. ...
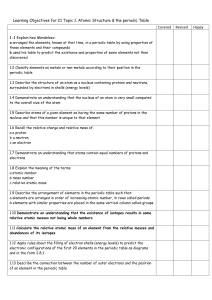
C2- Topic 1: Atomic structure and the periodic table. Assessable
... - arranged the elements, known at that time, in a periodic table by using properties of these elements and their compounds - used his table to predict the existence and properties of some elements not then discovered ...
... - arranged the elements, known at that time, in a periodic table by using properties of these elements and their compounds - used his table to predict the existence and properties of some elements not then discovered ...
C2 Topic 1 Can Do Sheet
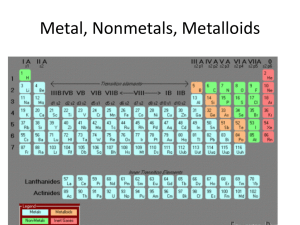
... a arranged the elements, known at that time, in a periodic table by using properties of these elements and their compounds b used his table to predict the existence and properties of some elements not then discovered 1.2 Classify elements as metals or non-metals according to their position in the pe ...
... a arranged the elements, known at that time, in a periodic table by using properties of these elements and their compounds b used his table to predict the existence and properties of some elements not then discovered 1.2 Classify elements as metals or non-metals according to their position in the pe ...
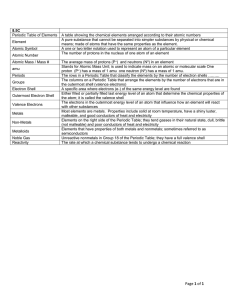
8.5C Vocabulary
... Periods Groups Electron Shell Outermost Electron Shell Valence Electrons Metals Non-Metals Metalloids Noble Gas Reactivity ...
... Periods Groups Electron Shell Outermost Electron Shell Valence Electrons Metals Non-Metals Metalloids Noble Gas Reactivity ...
Periodic Table Vocab page 7
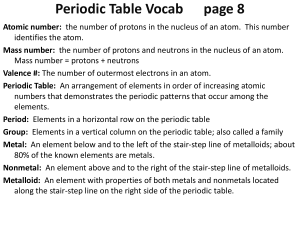
... Atomic number: the number of protons in the nucleus of an atom. This number identifies the atom. Mass number: the number of protons and neutrons in the nucleus of an atom. Mass number = protons + neutrons Valence #: The number of outermost electrons in an atom. Periodic Table: An arrangement of elem ...
... Atomic number: the number of protons in the nucleus of an atom. This number identifies the atom. Mass number: the number of protons and neutrons in the nucleus of an atom. Mass number = protons + neutrons Valence #: The number of outermost electrons in an atom. Periodic Table: An arrangement of elem ...
Periodic table
The periodic table is a tabular arrangement of the chemical elements, ordered by their atomic number (number of protons in the nucleus), electron configurations, and recurring chemical properties. The table also shows four rectangular blocks: s-, p- d- and f-block. In general, within one row (period) the elements are metals on the lefthand side, and non-metals on the righthand side.The rows of the table are called periods; the columns are called groups. Six groups (columns) have names as well as numbers: for example, group 17 elements are the halogens; and group 18, the noble gases. The periodic table can be used to derive relationships between the properties of the elements, and predict the properties of new elements yet to be discovered or synthesized. The periodic table provides a useful framework for analyzing chemical behavior, and is widely used in chemistry and other sciences.Although precursors exist, Dmitri Mendeleev is generally credited with the publication, in 1869, of the first widely recognized periodic table. He developed his table to illustrate periodic trends in the properties of the then-known elements. Mendeleev also predicted some properties of then-unknown elements that would be expected to fill gaps in this table. Most of his predictions were proved correct when the elements in question were subsequently discovered. Mendeleev's periodic table has since been expanded and refined with the discovery or synthesis of further new elements and the development of new theoretical models to explain chemical behavior.All elements from atomic numbers 1 (hydrogen) to 118 (ununoctium) have been discovered or reportedly synthesized, with elements 113, 115, 117, and 118 having yet to be confirmed. The first 94 elements exist naturally, although some are found only in trace amounts and were synthesized in laboratories before being found in nature. Elements with atomic numbers from 95 to 118 have only been synthesized in laboratories. It has been shown that einsteinium and fermium once occurred in nature but currently do not. Synthesis of elements having higher atomic numbers is being pursued. Numerous synthetic radionuclides of naturally occurring elements have also been produced in laboratories.