Chapter 9
... • ATP is unstable because the three phosphates in ATP are all negatively charged and repel one another. • When one phosphate group is removed by hydrolysis (the chemical breakdown of a compound due to reaction with water), a more stable molecule, ADP (adenosine diphosphate), results. • The change, f ...
... • ATP is unstable because the three phosphates in ATP are all negatively charged and repel one another. • When one phosphate group is removed by hydrolysis (the chemical breakdown of a compound due to reaction with water), a more stable molecule, ADP (adenosine diphosphate), results. • The change, f ...
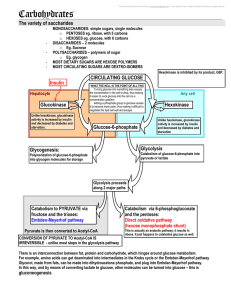
5 carbohydrates and the Krebs Cycle
... o It reacts with uridine diphosphoglucose to form uridine diphosphogalactose. o If you are defif\cient in the catalyzing enzyme, the galactose accumulates in the bloodstream and causes ...
... o It reacts with uridine diphosphoglucose to form uridine diphosphogalactose. o If you are defif\cient in the catalyzing enzyme, the galactose accumulates in the bloodstream and causes ...
Problem Set 1 - Berkeley MCB
... (B) helps to reduce blood glucose after a carbohydrate-rich meal. (C) is activated by the hormone insulin (D) is essential in the conversion of fatty acids to glucose. (E) requires the enzyme hexokinase. ...
... (B) helps to reduce blood glucose after a carbohydrate-rich meal. (C) is activated by the hormone insulin (D) is essential in the conversion of fatty acids to glucose. (E) requires the enzyme hexokinase. ...
Cellular Respiration CPB
... prokaryotes: same chain in cell membrane H-E e- move from 1 carrier protein to the next E is used to move H ions across membrane (ATP synthase) every rotation of ATPase phosphate group is added to A-P-P A-P-P~P absence of oxygen 2 ATP (glycolysis) presence of oxygen 34 more ATP g ...
... prokaryotes: same chain in cell membrane H-E e- move from 1 carrier protein to the next E is used to move H ions across membrane (ATP synthase) every rotation of ATPase phosphate group is added to A-P-P A-P-P~P absence of oxygen 2 ATP (glycolysis) presence of oxygen 34 more ATP g ...
Final Respiration
... • No ATP is made, but the NAD+ can keep glycolysis going to make a little ATP • 2 kinds of fermentation: Lactic acid fermentation and Alcoholic Fermentation ...
... • No ATP is made, but the NAD+ can keep glycolysis going to make a little ATP • 2 kinds of fermentation: Lactic acid fermentation and Alcoholic Fermentation ...
cellrespdiagrams
... • No ATP is made, but the NAD+ can keep glycolysis going to make a little ATP • 2 kinds of fermentation: Lactic acid fermentation and Alcoholic Fermentation ...
... • No ATP is made, but the NAD+ can keep glycolysis going to make a little ATP • 2 kinds of fermentation: Lactic acid fermentation and Alcoholic Fermentation ...
Final Respiration
... • No ATP is made, but the NAD+ can keep glycolysis going to make a little ATP • 2 kinds of fermentation: Lactic acid fermentation and Alcoholic Fermentation ...
... • No ATP is made, but the NAD+ can keep glycolysis going to make a little ATP • 2 kinds of fermentation: Lactic acid fermentation and Alcoholic Fermentation ...
Cell Respiration Outline | Date: Mitochondrion • Structure o Double
... Each turn of the cycle requires one acetyl CoA – must make 2 turns before glucose is completely oxidized ...
... Each turn of the cycle requires one acetyl CoA – must make 2 turns before glucose is completely oxidized ...
Cellular Respiration
... Series of enzymatic reactions that continually oxidize glucose in baby steps. A coenzyme (comes from a vitamin – what’s this again?) called NAD+ helps nibble, that’s right I said nibble, away electrons in small steps. Dehydrogenases play a role. What is it? What do dehydrogenases do to the pH of the ...
... Series of enzymatic reactions that continually oxidize glucose in baby steps. A coenzyme (comes from a vitamin – what’s this again?) called NAD+ helps nibble, that’s right I said nibble, away electrons in small steps. Dehydrogenases play a role. What is it? What do dehydrogenases do to the pH of the ...
File - Hope Christian College Parent and Student Portal
... •Energy is also needed for growth, cell division, movement and to get rid of waste products. •Energy comes in different forms but cells use chemical energy. •Chemical energy is stored in bonds or the connections that join the atoms to molecules. •Once a bond is broken energy is released. •Things lik ...
... •Energy is also needed for growth, cell division, movement and to get rid of waste products. •Energy comes in different forms but cells use chemical energy. •Chemical energy is stored in bonds or the connections that join the atoms to molecules. •Once a bond is broken energy is released. •Things lik ...
Chapter 4 Cellular Respiration
... NADH and FADH2 from Krebs Cycle are pumped by electron energy across the inner membrane (cristae) and creates a concentration difference. ...
... NADH and FADH2 from Krebs Cycle are pumped by electron energy across the inner membrane (cristae) and creates a concentration difference. ...
AP Biology Midterm Studyguide 2017
... F. Enzymes! 1. be sure to understand the enzyme catalyzed reaction graph (see below) 2. Terms: exergonic, endergonic, spontaneous, free energy, catabolism, anabolism 3. Active site, competitive inhibitors, allosteric reactions More Thoughts: A. Know the pathway of each of the reactants in P and R (r ...
... F. Enzymes! 1. be sure to understand the enzyme catalyzed reaction graph (see below) 2. Terms: exergonic, endergonic, spontaneous, free energy, catabolism, anabolism 3. Active site, competitive inhibitors, allosteric reactions More Thoughts: A. Know the pathway of each of the reactants in P and R (r ...
Biochemistry - Bonham Chemistry
... Cellular Respiration: the big picture • process in which cells consume O2 and produce CO2 ...
... Cellular Respiration: the big picture • process in which cells consume O2 and produce CO2 ...
GLYCOLYSIS (1).
... • Glycolysis of glucose to provide ATP anaerobically is especially important, because skeletal muscles can perform under anoxic conditions. • Cardiac muscles have low glycolytic activity. ...
... • Glycolysis of glucose to provide ATP anaerobically is especially important, because skeletal muscles can perform under anoxic conditions. • Cardiac muscles have low glycolytic activity. ...
C483 Final Exam Study Guide The final will be held in Chemistry
... molecule that you store in your liver. Circle the pathways/cycles below that are part of this overall transformation. Cross out any that are not. Gluconeogenesis, pentose phosphate pathway, glycogen synthesis, glycolysis, citric acid cycle B. Trace the metabolic path of this glutamate molecule throu ...
... molecule that you store in your liver. Circle the pathways/cycles below that are part of this overall transformation. Cross out any that are not. Gluconeogenesis, pentose phosphate pathway, glycogen synthesis, glycolysis, citric acid cycle B. Trace the metabolic path of this glutamate molecule throu ...
GLYCOLYSIS
... • Glycolysis of glucose to provide ATP anaerobically is especially important, because skeletal muscles can perform under anoxic conditions. • Cardiac muscles have low glycolytic activity. ...
... • Glycolysis of glucose to provide ATP anaerobically is especially important, because skeletal muscles can perform under anoxic conditions. • Cardiac muscles have low glycolytic activity. ...
Anaerobic Respiration - University of Indianapolis
... During heavy exercise, ATP production will switch from aerobic respiration to anerobic respiration ...
... During heavy exercise, ATP production will switch from aerobic respiration to anerobic respiration ...
Alcoholic fermentation
... …………………….. back to NAD+ so that the energy yielding phase of glycolysis can continue. In yeast, pyruvate is decarboxylated to ETHANAL (…..C), releasing …………….. . The enzyme alcohol dehydrogenase then ……………….. ETHANAL to ETHANOL (…..C), at the same time ………………… NADH back to ……………. . CH3CHO + NADH ...
... …………………….. back to NAD+ so that the energy yielding phase of glycolysis can continue. In yeast, pyruvate is decarboxylated to ETHANAL (…..C), releasing …………….. . The enzyme alcohol dehydrogenase then ……………….. ETHANAL to ETHANOL (…..C), at the same time ………………… NADH back to ……………. . CH3CHO + NADH ...
Metabolism08
... 4. Electron Transport Chain: This pathway produces most of the ATP available from glucose ...
... 4. Electron Transport Chain: This pathway produces most of the ATP available from glucose ...
Glycolysis
Glycolysis (from glycose, an older term for glucose + -lysis degradation) is the metabolic pathway that converts glucose C6H12O6, into pyruvate, CH3COCOO− + H+. The free energy released in this process is used to form the high-energy compounds ATP (adenosine triphosphate) and NADH (reduced nicotinamide adenine dinucleotide).Glycolysis is a determined sequence of ten enzyme-catalyzed reactions. The intermediates provide entry points to glycolysis. For example, most monosaccharides, such as fructose and galactose, can be converted to one of these intermediates. The intermediates may also be directly useful. For example, the intermediate dihydroxyacetone phosphate (DHAP) is a source of the glycerol that combines with fatty acids to form fat.Glycolysis is an oxygen independent metabolic pathway, meaning that it does not use molecular oxygen (i.e. atmospheric oxygen) for any of its reactions. However the products of glycolysis (pyruvate and NADH + H+) are sometimes disposed of using atmospheric oxygen. When molecular oxygen is used in the disposal of the products of glycolysis the process is usually referred to as aerobic, whereas if the disposal uses no oxygen the process is said to be anaerobic. Thus, glycolysis occurs, with variations, in nearly all organisms, both aerobic and anaerobic. The wide occurrence of glycolysis indicates that it is one of the most ancient metabolic pathways. Indeed, the reactions that constitute glycolysis and its parallel pathway, the pentose phosphate pathway, occur metal-catalyzed under the oxygen-free conditions of the Archean oceans, also in the absence of enzymes. Glycolysis could thus have originated from chemical constraints of the prebiotic world.Glycolysis occurs in most organisms in the cytosol of the cell. The most common type of glycolysis is the Embden–Meyerhof–Parnas (EMP pathway), which was discovered by Gustav Embden, Otto Meyerhof, and Jakub Karol Parnas. Glycolysis also refers to other pathways, such as the Entner–Doudoroff pathway and various heterofermentative and homofermentative pathways. However, the discussion here will be limited to the Embden–Meyerhof–Parnas pathway.The entire glycolysis pathway can be separated into two phases: The Preparatory Phase – in which ATP is consumed and is hence also known as the investment phase The Pay Off Phase – in which ATP is produced.↑ ↑ 2.0 2.1 ↑ ↑ ↑ ↑ ↑ ↑