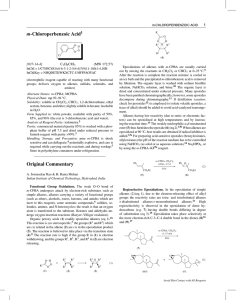
Chloroperbenzoic_aci..
... reagent of choice for laboratory-scale reactions. For large-scale epoxidations the cheaper PAA is preferred. The highly reactive TFPAA is used for unreactive and heat-sensitive substrates; its reactivity permits the use of low reaction temperatures. The recently introduced reagent magnesium monoperp ...
... reagent of choice for laboratory-scale reactions. For large-scale epoxidations the cheaper PAA is preferred. The highly reactive TFPAA is used for unreactive and heat-sensitive substrates; its reactivity permits the use of low reaction temperatures. The recently introduced reagent magnesium monoperp ...
IR Spectroscopy and Mass Spectroscopy
... Variations in C=O Absorption ¾ The position of the absorption band depends on electron delocalization, the electronic effect of neighboring substituents, and hydrogen bonding. ¾ As conjugation lower the C=C stretching frequency, it also lower the C=O absorption frequency to ~ 1680 cm-1. ...
... Variations in C=O Absorption ¾ The position of the absorption band depends on electron delocalization, the electronic effect of neighboring substituents, and hydrogen bonding. ¾ As conjugation lower the C=C stretching frequency, it also lower the C=O absorption frequency to ~ 1680 cm-1. ...
1012_4th Exam_1020619 - NTOU-Chem
... 3) The binding energy per nucleon is largest for ________. A) 1H B) 59Co C) 3He ...
... 3) The binding energy per nucleon is largest for ________. A) 1H B) 59Co C) 3He ...
Example 7.1: The following decomposition was studied at a given
... The rate laws arise from the reaction mechanism i.e. how the molecules actually break and form bonds. The orders for each reactant must be determined experimentally. ...
... The rate laws arise from the reaction mechanism i.e. how the molecules actually break and form bonds. The orders for each reactant must be determined experimentally. ...
Number of students performing at
... The reaction with HBr would result in substitution and formation of the corresponding alkyl bromide. The process could occur following either an SN1 or an SN2 mechanism. Based on the structure of each of the alcohols, one can make a prediction on the mechanism of its reaction with HBr, as shown abov ...
... The reaction with HBr would result in substitution and formation of the corresponding alkyl bromide. The process could occur following either an SN1 or an SN2 mechanism. Based on the structure of each of the alcohols, one can make a prediction on the mechanism of its reaction with HBr, as shown abov ...
Chemical Equations - Salem Community Schools
... Balancing an Equation Is the equation balanced now? Two sodium atoms are on each side. How many oxygen atoms are on each side? You should be able to find four on each side. How about hydrogen atoms? Now two are on each side. Because one carbon atom is still on each side, the entire equation is balan ...
... Balancing an Equation Is the equation balanced now? Two sodium atoms are on each side. How many oxygen atoms are on each side? You should be able to find four on each side. How about hydrogen atoms? Now two are on each side. Because one carbon atom is still on each side, the entire equation is balan ...
Transition Metal Catalyzed Carbon
... Steric Tuning of Silylacetylenes and Chiral Phosphine Ligands for Rhodium-Catalyzed Asymmetric Conjugated Alkynylation of Enones Hayashi et al. J. Am. Chem. Soc. 2008, 130, 1576. Under one of the standard reaction conditions for the rhodium-catalyzed asymmetric addition... ...
... Steric Tuning of Silylacetylenes and Chiral Phosphine Ligands for Rhodium-Catalyzed Asymmetric Conjugated Alkynylation of Enones Hayashi et al. J. Am. Chem. Soc. 2008, 130, 1576. Under one of the standard reaction conditions for the rhodium-catalyzed asymmetric addition... ...
Manganese-Catalyzed Carbonylation of Alkyl Iodides
... useful transformations when generated and used in situ, hence a number of methods for their formation have been developed. 1' 2 Although highly reactive in solution, arynes can be stabilized by electron rich metal fragments. This stabilization can be attributed to several factors: 1) the aryne is le ...
... useful transformations when generated and used in situ, hence a number of methods for their formation have been developed. 1' 2 Although highly reactive in solution, arynes can be stabilized by electron rich metal fragments. This stabilization can be attributed to several factors: 1) the aryne is le ...
Chapter 13 Organic Chemistry
... while the highly branched alkane C8H18 causes little knocking and is assigned an octane rating of 100. A gasoline with an octane rating of 87 causes the same knocking as a mixture that is 87% in the branched alkane and 13% of the straight chain alkane. Alkenes are organic compounds that contain carb ...
... while the highly branched alkane C8H18 causes little knocking and is assigned an octane rating of 100. A gasoline with an octane rating of 87 causes the same knocking as a mixture that is 87% in the branched alkane and 13% of the straight chain alkane. Alkenes are organic compounds that contain carb ...
Iron(II) Chloride–1,1′-Binaphthyl-2,2′-diamine
... The reaction was completely suppressed by adding one equivalent of TEMPO (with respect to FeCl2), a radical trapping agent, to the reaction mixture. These results indicate that a radical intermediate is most likely involved in the initial steps of the domino transformation. This explains the observe ...
... The reaction was completely suppressed by adding one equivalent of TEMPO (with respect to FeCl2), a radical trapping agent, to the reaction mixture. These results indicate that a radical intermediate is most likely involved in the initial steps of the domino transformation. This explains the observe ...
Synthetic applications of ortho esters
... In contrast to acetal derivatives of carbonyl compounds, ortho esters have found surprisingly limited use in organic synthesis [1]. Since ortho esters are among the few carboxylic acid protective groups that demonstrate a high level of stability toward strong nucleophiles and bases, most current app ...
... In contrast to acetal derivatives of carbonyl compounds, ortho esters have found surprisingly limited use in organic synthesis [1]. Since ortho esters are among the few carboxylic acid protective groups that demonstrate a high level of stability toward strong nucleophiles and bases, most current app ...
Spotlight 106 Oxalic Acid: A Very Useful Brønsted Acid in Organic Synthesis SYNLETT
... to b-keto phosphonates. b-Keto phosphonates are excellent reagents for homologation of aldehydes and ketones to a,b-unsaturated aldehydes and ketones via the Wadsworth–Emmons–Horner reaction.12 (F) Alcohols have been efficiently converted into alkenes either by heating with anhydrous oxalic acid or ...
... to b-keto phosphonates. b-Keto phosphonates are excellent reagents for homologation of aldehydes and ketones to a,b-unsaturated aldehydes and ketones via the Wadsworth–Emmons–Horner reaction.12 (F) Alcohols have been efficiently converted into alkenes either by heating with anhydrous oxalic acid or ...
Stoichiometry of Ozonation of Environmentally
... organics in a well-defined stoichiometric ratio, with one carbon-carbon double bond consumed per ozone molecule reacted. However, this work demonstrates that a single ozone molecule can effectively destroy several double bonds in dilute solutions of monoterpenes and unsaturated fatty acids via a cha ...
... organics in a well-defined stoichiometric ratio, with one carbon-carbon double bond consumed per ozone molecule reacted. However, this work demonstrates that a single ozone molecule can effectively destroy several double bonds in dilute solutions of monoterpenes and unsaturated fatty acids via a cha ...
Carboxylic Acids, Amines, and Amides
... • We have seen some examples of stereoisomers in the past: – Geometric isomers (cis and trans) ...
... • We have seen some examples of stereoisomers in the past: – Geometric isomers (cis and trans) ...
An Anionic Dimer of Cyclopentadienyl
... and catalysis, to novel chemistry of molecular systems [1]. Particularly, complexes having two widely divergent metals, one a Lewis-acid early transition metal and the other an electronrich late transition metal are promising candidates for new stoichiometric and catalytic reactions [2,3] and they s ...
... and catalysis, to novel chemistry of molecular systems [1]. Particularly, complexes having two widely divergent metals, one a Lewis-acid early transition metal and the other an electronrich late transition metal are promising candidates for new stoichiometric and catalytic reactions [2,3] and they s ...
Alkyl Halides02
... A nucleophilic substitution reaction with 2nd order kinetics is called an SN2 reaction. SN2 = Substitution, Nucleophilic, 2nd order (called 'bimolecular'). An essential feature of an SN2 mechanism is that the reaction occurs in a single step, without intermediates, i.e., as the Nu:- bonds, the X lea ...
... A nucleophilic substitution reaction with 2nd order kinetics is called an SN2 reaction. SN2 = Substitution, Nucleophilic, 2nd order (called 'bimolecular'). An essential feature of an SN2 mechanism is that the reaction occurs in a single step, without intermediates, i.e., as the Nu:- bonds, the X lea ...
2. The Magic of Chemical Reactions
... The chemical formula of POP is ------. An electric bulb has a filament made of element called ------. The yellow oily leftover stains turn red / orange because of ------. Chemical reaction involves breaking and making of the bonds between the atoms to produce ------ ------. The chemical reaction dur ...
... The chemical formula of POP is ------. An electric bulb has a filament made of element called ------. The yellow oily leftover stains turn red / orange because of ------. Chemical reaction involves breaking and making of the bonds between the atoms to produce ------ ------. The chemical reaction dur ...
Microwave-Assisted Esterification of N -Acetyl-L-Phenylalanine Using Modified Mukaiyama s Reagents: A New Approach Involving Ionic Liquids
... because 2-fluoro-onium is too reactive and is known to react with the alcohol directly (since it is also in excess) [42-43]. The fact that [2-ClMePy]I produced a higher yield (entry 3 in Table 1) than other IL-type coupling reagents, is probably due to its smaller anion size (I-) than others (such a ...
... because 2-fluoro-onium is too reactive and is known to react with the alcohol directly (since it is also in excess) [42-43]. The fact that [2-ClMePy]I produced a higher yield (entry 3 in Table 1) than other IL-type coupling reagents, is probably due to its smaller anion size (I-) than others (such a ...
Asymmetric induction

Asymmetric induction (also enantioinduction) in stereochemistry describes the preferential formation in a chemical reaction of one enantiomer or diastereoisomer over the other as a result of the influence of a chiral feature present in the substrate, reagent, catalyst or environment. Asymmetric induction is a key element in asymmetric synthesis.Asymmetric induction was introduced by Hermann Emil Fischer based on his work on carbohydrates. Several types of induction exist.Internal asymmetric induction makes use of a chiral center bound to the reactive center through a covalent bond and remains so during the reaction. The starting material is often derived from chiral pool synthesis. In relayed asymmetric induction the chiral information is introduced in a separate step and removed again in a separate chemical reaction. Special synthons are called chiral auxiliaries. In external asymmetric induction chiral information is introduced in the transition state through a catalyst of chiral ligand. This method of asymmetric synthesis is economically most desirable.























