Learning materials
... 8. Dehydration of alcohols 9. Synthesis of ethers 10. Solvents in organic chemistry ...
... 8. Dehydration of alcohols 9. Synthesis of ethers 10. Solvents in organic chemistry ...
Organometallics - X-Ray - University of Kentucky
... and DFT computational studies. It does not appear that 4 possesses agostic interactions in solution. High oxidation state transition metal complexes containing double metal-carbon bonds (usually termed Schrock1 carbenes or alkylidenes) have attracted significant attention,2 in part because of their ...
... and DFT computational studies. It does not appear that 4 possesses agostic interactions in solution. High oxidation state transition metal complexes containing double metal-carbon bonds (usually termed Schrock1 carbenes or alkylidenes) have attracted significant attention,2 in part because of their ...
aminepp - Knockhardy
... Addition of aqueous sodium hydroxide liberates the free base from its salt C6H5NH3+Cl¯(aq) ...
... Addition of aqueous sodium hydroxide liberates the free base from its salt C6H5NH3+Cl¯(aq) ...
Reactions of carboxymethylalginic acid with some N
... compounds, and the more heat appears, the more carboxyl groups that can react with a nucleophile is formed. Therefore, when the temperature rises from 80 to 100°C Cs values are increased by more than 5-fold from 0.07 to 0.38 moles per monosaccharide fragment. In the reaction products of H-form with ...
... compounds, and the more heat appears, the more carboxyl groups that can react with a nucleophile is formed. Therefore, when the temperature rises from 80 to 100°C Cs values are increased by more than 5-fold from 0.07 to 0.38 moles per monosaccharide fragment. In the reaction products of H-form with ...
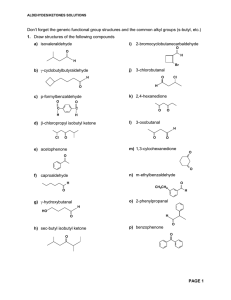
Aldehydes/Ketones Solutions
... mechanisms required but show all reagents and intermediate products formed. More than one step may be necessary a) O O C H ...
... mechanisms required but show all reagents and intermediate products formed. More than one step may be necessary a) O O C H ...
CH - YSU.edu
... 13. (5 pts) Explain using diagrams why cis-1,3-dimethylcyclohexane is thermodynamically more stable than trans-1,3-dimethylcyclohexane. ...
... 13. (5 pts) Explain using diagrams why cis-1,3-dimethylcyclohexane is thermodynamically more stable than trans-1,3-dimethylcyclohexane. ...
Dicyanomethylenedihydrofuran photorefractive materials
... First observed in inorganic crystals such as LiNbO3 and LiTaO3 in 1969 1, the PR effect is a reversible refractive index modulation process induced by light and an electric field in a material. The PR effect has promising applications including optical data storage, phase conjugation and optical pro ...
... First observed in inorganic crystals such as LiNbO3 and LiTaO3 in 1969 1, the PR effect is a reversible refractive index modulation process induced by light and an electric field in a material. The PR effect has promising applications including optical data storage, phase conjugation and optical pro ...
Cl + CH3OH * HCl + CH2OH
... Where kb(i) is the liquid-phase rate coefficient for reaction of NO3 with organic species (i) with concentration [HC], Dl its diffusion coefficient through the organic matrix and H its solubility. A rough estimate for a generic uptake coefficient for NO3 uptake to saturated alcohols or carbonyls can ...
... Where kb(i) is the liquid-phase rate coefficient for reaction of NO3 with organic species (i) with concentration [HC], Dl its diffusion coefficient through the organic matrix and H its solubility. A rough estimate for a generic uptake coefficient for NO3 uptake to saturated alcohols or carbonyls can ...
Terrahedron Letters. Vo1.32, No.43, pi 6089
... Nicolaou has reported that similar substituted epoxy alcohols give predominately cyclization rather than that of a 6-endo cyclization. ...
... Nicolaou has reported that similar substituted epoxy alcohols give predominately cyclization rather than that of a 6-endo cyclization. ...
Ring-Opening Metathesis Polymerization of Norbornene Catalyzed
... Ru-carbene and Ru-vinylidene complexes have been proven to be highly efficient catalysts for a variety of olefin transformations. These include examples of ring-opening metathesis polymerization (ROMP) [1-7], ring-closing metathesis (RCM) [8,9], polycyclization reactions [9,10] and synthesis of natu ...
... Ru-carbene and Ru-vinylidene complexes have been proven to be highly efficient catalysts for a variety of olefin transformations. These include examples of ring-opening metathesis polymerization (ROMP) [1-7], ring-closing metathesis (RCM) [8,9], polycyclization reactions [9,10] and synthesis of natu ...
Ch. 16 Study Guide
... 16. The reaction quotient, Qc , is expressed as concentration of products over concentration of reactants, with each substance raised to its stoichiometric power. There is also a Qp , of course. Q is for a specific set of reaction conditions that may or may not be at equilibrium. 17. If Q > K (conce ...
... 16. The reaction quotient, Qc , is expressed as concentration of products over concentration of reactants, with each substance raised to its stoichiometric power. There is also a Qp , of course. Q is for a specific set of reaction conditions that may or may not be at equilibrium. 17. If Q > K (conce ...
Reduction of Aldehydes and Ketones to Corresponding
... Isolated yields are based on single experiment and yields were not optimized Boiling point. *The reaction was carried out under reflux condition ...
... Isolated yields are based on single experiment and yields were not optimized Boiling point. *The reaction was carried out under reflux condition ...
Chapter 24. Amines
... delocalized by interaction with the aromatic ring electron system and are less able to accept H+ than are alkylamines ...
... delocalized by interaction with the aromatic ring electron system and are less able to accept H+ than are alkylamines ...
f8560d95306293b
... • The oxygen atom in alcohols, ethers and epoxides is sp3 hybridized. Alcohols and ethers have a bent shape like that in H2O. • The bond angle around the O atom in an alcohol or ether is similar to the tetrahedral bond angle of 109.5°. • Because the O atom is much more electronegative than carbon o ...
... • The oxygen atom in alcohols, ethers and epoxides is sp3 hybridized. Alcohols and ethers have a bent shape like that in H2O. • The bond angle around the O atom in an alcohol or ether is similar to the tetrahedral bond angle of 109.5°. • Because the O atom is much more electronegative than carbon o ...
Carboxylic Acid Derivatives and Nucleophilic Acyl Substitution
... groups • More electrophilic carbonyl groups are more reactive to addition (acyl halides are most reactive, amides are least) • The intermediate with the best leaving group decomposes fastest ...
... groups • More electrophilic carbonyl groups are more reactive to addition (acyl halides are most reactive, amides are least) • The intermediate with the best leaving group decomposes fastest ...
CH 17 Study Guide with answer Key
... the indicated substance is added. Identify one other way in which the reaction could be shifted in the same direction you indicated. (Hint: There may be more than one way to do this.) 3. Reaction: N2(g) 3H2(g) 2NH3(g); NH3 added ...
... the indicated substance is added. Identify one other way in which the reaction could be shifted in the same direction you indicated. (Hint: There may be more than one way to do this.) 3. Reaction: N2(g) 3H2(g) 2NH3(g); NH3 added ...
Regiospecificity according to Markovnikov
... on carbon, Pd/C) converts alkynes to alkanes (complete reduction) • The addition of the first equivalent of H2 produces an alkene, which is more reactive than the alkyne so the alkene is not observed ...
... on carbon, Pd/C) converts alkynes to alkanes (complete reduction) • The addition of the first equivalent of H2 produces an alkene, which is more reactive than the alkyne so the alkene is not observed ...
Asymmetric induction

Asymmetric induction (also enantioinduction) in stereochemistry describes the preferential formation in a chemical reaction of one enantiomer or diastereoisomer over the other as a result of the influence of a chiral feature present in the substrate, reagent, catalyst or environment. Asymmetric induction is a key element in asymmetric synthesis.Asymmetric induction was introduced by Hermann Emil Fischer based on his work on carbohydrates. Several types of induction exist.Internal asymmetric induction makes use of a chiral center bound to the reactive center through a covalent bond and remains so during the reaction. The starting material is often derived from chiral pool synthesis. In relayed asymmetric induction the chiral information is introduced in a separate step and removed again in a separate chemical reaction. Special synthons are called chiral auxiliaries. In external asymmetric induction chiral information is introduced in the transition state through a catalyst of chiral ligand. This method of asymmetric synthesis is economically most desirable.