Topic 4 - Lloyd Crosby
... c. A complex ion is an ion in which a ligand is covalently bound to a metal. d. A ligand is any molecule or ion connected to the central ion or atom of a complex by means of a coordinate covalent bond. e. Coordination number The coordination number is the total number of bonds the metal ion forms wi ...
... c. A complex ion is an ion in which a ligand is covalently bound to a metal. d. A ligand is any molecule or ion connected to the central ion or atom of a complex by means of a coordinate covalent bond. e. Coordination number The coordination number is the total number of bonds the metal ion forms wi ...
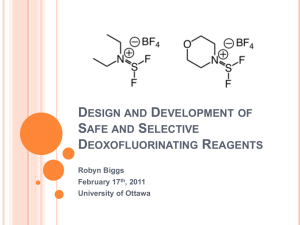
Design and Development of Safe and Selective Deoxofluorinating
... Ease of handling Selective Stable ...
... Ease of handling Selective Stable ...
Chapter 22 Alpha Substitution and Condensations of Enols
... • When C=C is conjugated with C=O, 1,2-addition or 1,4-addition may occur. • A 1,4-addition of an enolate ion is called the Michael reaction. ...
... • When C=C is conjugated with C=O, 1,2-addition or 1,4-addition may occur. • A 1,4-addition of an enolate ion is called the Michael reaction. ...
CHAPTER 1 Synthesis of amides using Lewis acid catalyst: Iodine
... the right, because the unification of these two functional groups does not occur spontaneously at ambient temperature, with the necessary elimination of water only taking place at high temperatures (e.g. >200 ºC),29 conditions typically detrimental to the integrity of the substrates. For this reason ...
... the right, because the unification of these two functional groups does not occur spontaneously at ambient temperature, with the necessary elimination of water only taking place at high temperatures (e.g. >200 ºC),29 conditions typically detrimental to the integrity of the substrates. For this reason ...
Şenol, O.İ., Viljava, T.-R., Krause, AOI
... the deesterification reaction yields carboxylic acids and methanol. The water required for the reaction may be supplied by the dehydration of the alcohols in path I. The formed carboxylic acids are either reduced to alcohol releasing a mole of water or decarboxylated to alkenes followed by hydrogena ...
... the deesterification reaction yields carboxylic acids and methanol. The water required for the reaction may be supplied by the dehydration of the alcohols in path I. The formed carboxylic acids are either reduced to alcohol releasing a mole of water or decarboxylated to alkenes followed by hydrogena ...
College Chemistry I PHS 1025 Fall 2012 Practice Exam 3A
... 47) Which one of the following compounds is insoluble in water? A) Rb2CO3 B) PbSO4 C) K2SO4 48) In which compound is the oxidation state of hydrogen not +1? A) Na2HSO4 B) NaH C) H2O ...
... 47) Which one of the following compounds is insoluble in water? A) Rb2CO3 B) PbSO4 C) K2SO4 48) In which compound is the oxidation state of hydrogen not +1? A) Na2HSO4 B) NaH C) H2O ...
When 1°, 2°, aromatic amines or aryl amines . (Rand
... vigorously at room or high temperature to yield phenols and this is the reason why these salts are used immediately after preparation. Ar-N2+x- ...
... vigorously at room or high temperature to yield phenols and this is the reason why these salts are used immediately after preparation. Ar-N2+x- ...
Alkyl Aryl Ether Bond Formation with PhenoFluor
... bond formation is appealing and orthogonal to other crosscoupling approaches. The Mitsunobu reaction has been developed for this purpose, and a large substrate scope has been demonstrated.[6, 7] However, several substrate classes, such as salicylaldehydes, are not tolerated, and general alcohol–alco ...
... bond formation is appealing and orthogonal to other crosscoupling approaches. The Mitsunobu reaction has been developed for this purpose, and a large substrate scope has been demonstrated.[6, 7] However, several substrate classes, such as salicylaldehydes, are not tolerated, and general alcohol–alco ...
Chapter 14
... A) oxygen is a reactant in combustion and concentration of oxygen is higher in pure oxygen than is in air. B) oxygen is a catalyst for combustion. C) oxygen is a product of combustion. D) nitrogen is a product of combustion and the system reaches equilibrium at a lower temperature. E) nitrogen is a ...
... A) oxygen is a reactant in combustion and concentration of oxygen is higher in pure oxygen than is in air. B) oxygen is a catalyst for combustion. C) oxygen is a product of combustion. D) nitrogen is a product of combustion and the system reaches equilibrium at a lower temperature. E) nitrogen is a ...
Lecture 31 Homogeneous catalysis
... The homogeneous catalyst precursors are added in the reaction system in different forms and are transformed into the active form insitu. During one catalytic cycle, the catalyst may pass through several intermediate forms and finally produce the products. After end of each catalytic cycle, the catal ...
... The homogeneous catalyst precursors are added in the reaction system in different forms and are transformed into the active form insitu. During one catalytic cycle, the catalyst may pass through several intermediate forms and finally produce the products. After end of each catalytic cycle, the catal ...
Unit_4_Notes_
... rates can range from microseconds to millions of years. There are 5 main factors that affect reaction rates – the book leaves out the first to be understood o Nature of the reactants: large, complex molecules tend to have slower reaction rates than smaller, simpler molecules. They have more area a ...
... rates can range from microseconds to millions of years. There are 5 main factors that affect reaction rates – the book leaves out the first to be understood o Nature of the reactants: large, complex molecules tend to have slower reaction rates than smaller, simpler molecules. They have more area a ...
4.2- Reaction Stoichiometry Reaction Stoichiometry
... 4.3 –Limiting reactant, Theoretical and percent Yield Limiting reactant or reagent(L.R)- The reactant that makes the least amount of the product and is completely consumed in the reaction that limits the amount of the product in a chemical reaction. Excess Reactant- Any reactant that occurs in a qu ...
... 4.3 –Limiting reactant, Theoretical and percent Yield Limiting reactant or reagent(L.R)- The reactant that makes the least amount of the product and is completely consumed in the reaction that limits the amount of the product in a chemical reaction. Excess Reactant- Any reactant that occurs in a qu ...
(General Equilibrium) Part 1
... As soon as you start the reaction, a little bit of the product goes back to being reactants. With time, the concentration of reactant decreases and the concentration of product increases until both concentrations level off at constant, equilibrium values. Reactants start high and go ___________ . Pr ...
... As soon as you start the reaction, a little bit of the product goes back to being reactants. With time, the concentration of reactant decreases and the concentration of product increases until both concentrations level off at constant, equilibrium values. Reactants start high and go ___________ . Pr ...
CHAPTER 21 PHENOLS AND ARYL HALIDES
... Aryl halides and vinylic halides are relatively unreactive toward nucleophilic substitution under conditions that give facile nucleophilic substitution with alkyl halides. Reason: (1) Phenyl cations are very unstable. (2) Halogen bonds of aryl (and vinylic) halides are shorter and stronger than tho ...
... Aryl halides and vinylic halides are relatively unreactive toward nucleophilic substitution under conditions that give facile nucleophilic substitution with alkyl halides. Reason: (1) Phenyl cations are very unstable. (2) Halogen bonds of aryl (and vinylic) halides are shorter and stronger than tho ...
100 Problems and Exercises in Organometallic Chemistry Anil J. Elias
... (Fischer E. O. et al., J. Organomet. Chem., 1972, 35, 647 & 1974, 81, C23) 37. Write the structure of the most appropriate reagent and conditions for carrying out the following reactions resulting in the highest possible yields. Indicate ...
... (Fischer E. O. et al., J. Organomet. Chem., 1972, 35, 647 & 1974, 81, C23) 37. Write the structure of the most appropriate reagent and conditions for carrying out the following reactions resulting in the highest possible yields. Indicate ...
Review
... that Br2 would. Also unlike Br2, it goes only for the benzylic/allylic position, and doesn’t react significantly with tertiary carbons. Again, if you do this reaction at the allylic position, a mixture of products is possible based on different resonance forms. Reactions with Carbanions Again, a neg ...
... that Br2 would. Also unlike Br2, it goes only for the benzylic/allylic position, and doesn’t react significantly with tertiary carbons. Again, if you do this reaction at the allylic position, a mixture of products is possible based on different resonance forms. Reactions with Carbanions Again, a neg ...
Carbohydrates
... break down cellulose. In humans it is primarily bulk or roughage. Chitin is a structural polysaccharide found in the exoskeletons of some insects. Chitin is a leather like structural substance that eventually hardens when it is shed. Chitin is often used in medicine for sutures because it is both st ...
... break down cellulose. In humans it is primarily bulk or roughage. Chitin is a structural polysaccharide found in the exoskeletons of some insects. Chitin is a leather like structural substance that eventually hardens when it is shed. Chitin is often used in medicine for sutures because it is both st ...
MHS Student Guide to Organic Chemistry
... compounds and then shows how to combine these names to represent the more complicated compounds. The simple organic compound are called “hydrocarbons” because they contain only carbon and hydrogen atoms. The simple groups of hydrocarbons are called the alkanes. Alkanes consist of straight chains of ...
... compounds and then shows how to combine these names to represent the more complicated compounds. The simple organic compound are called “hydrocarbons” because they contain only carbon and hydrogen atoms. The simple groups of hydrocarbons are called the alkanes. Alkanes consist of straight chains of ...
Organic Chemistry Durham School Board March
... Some examples of common organic compounds include methane (CH4), propane (C3H8), and butane, (C4H10). Organic compounds are also the main component of plants and animals, and account for the wide diversity of living organisms on our planet. The main reason for this diversity is the unique bonding na ...
... Some examples of common organic compounds include methane (CH4), propane (C3H8), and butane, (C4H10). Organic compounds are also the main component of plants and animals, and account for the wide diversity of living organisms on our planet. The main reason for this diversity is the unique bonding na ...
Asymmetric induction

Asymmetric induction (also enantioinduction) in stereochemistry describes the preferential formation in a chemical reaction of one enantiomer or diastereoisomer over the other as a result of the influence of a chiral feature present in the substrate, reagent, catalyst or environment. Asymmetric induction is a key element in asymmetric synthesis.Asymmetric induction was introduced by Hermann Emil Fischer based on his work on carbohydrates. Several types of induction exist.Internal asymmetric induction makes use of a chiral center bound to the reactive center through a covalent bond and remains so during the reaction. The starting material is often derived from chiral pool synthesis. In relayed asymmetric induction the chiral information is introduced in a separate step and removed again in a separate chemical reaction. Special synthons are called chiral auxiliaries. In external asymmetric induction chiral information is introduced in the transition state through a catalyst of chiral ligand. This method of asymmetric synthesis is economically most desirable.