* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download IR Spectroscopy and Mass Spectroscopy
Survey
Document related concepts
Transcript
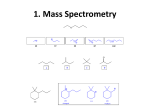
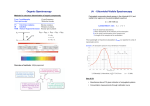
KOT 222 ORGANIC CHEMISTRY II CHAPTER 12 INFRARED SPECTROSCOPY and MASS SPECTROSCOPY Part II Infrared Spectroscopy IR Spectroscopy of Alcohols and Amines ¾ The O-H and N-H bonds are strong and stiff to give vibration at higher frequencies ~ 3300 cm-1. alcohol O-H acid O-H amine N-H R O 3300 cm-1, broad 3000 cm-1, broad 3300 cm-1, broad with spikes H H R N R'' H H R N R' R N R' IR Spectrum of Alcohol ¾The broad shape at around 3300 cm-1 is due to the diverse nature of the hydrogen bonding interactions of alcohol molecules. ¾Band centered near 1050 cm-1 is assigned to C-O stretching. Other functional groups also absorb at 1000 – 1200 cm-1 !! The position and the breadth of the O–H absorption band depend on the concentration of the solution It is easier to stretch an O–H bond if it is hydrogen bonded IR Spectrum of Amine Depends on no. of N-H bond 2o amine 1o amine IR Spectroscopy of Carbonyl Compounds ¾ C=O double bond has a large dipole moment which produce intense absorption. ¾ The C=O stretching vibrations of simple ketones, aldehydes, and carboxylic acids occur at frequencies around 1710 cm-1. Unusual strong hydrogen bonding in carboxylic acids, shift the broad O-H absorption band to about 3000 cm-1. Participation of C=O in hydrogen bonding also results in broadening of it absorption band. Variations in C=O Absorption ¾ The position of the absorption band depends on electron delocalization, the electronic effect of neighboring substituents, and hydrogen bonding. ¾ As conjugation lower the C=C stretching frequency, it also lower the C=O absorption frequency to ~ 1680 cm-1. The weak C=C absorption band may not appear. If presence, however, can be inferred from: its effect on the C=O absorption frequency. absorption of =C-H above 3000 cm-1. The C=O group of an amide absorbs at an even lower frequency, 1640-1680 cm-1. For amide: • strong C=O absorption. • prominent N-H stretching absorption. For alkene: • weak C=C absorption. • appearance of unsaturated C-H stretching. Carbonyl absorption above 1710 cm-1 ¾ The C=O of an ester absorbs at a higher frequency, ~1730-1740 cm-1. ¾ Carbonyl groups in small rings (5 C’s or less) absorb at an even higher frequency. Absorption of C-N Bonds ¾ Carbon-nitrogen bonds are more polar than carbon-carbon bonds, thus give stronger absorption. ¾ C-N single bonds absorb around 1200 cm-1. • Close to C-C and C-O absorptions ¾C=N double bonds absorb at 1660 cm-1. • Same region as C=C, but stronger absorption. ¾C≡C triple bonds strongly absorb just above 2200 cm-1. • C≡C signal is much weaker and is just below 2200 cm-1 IR Spectrum of Nitrile Summary of IR Spectroscopy IR spectrum can: 1. Indicate the functional group in the compound. 2. Show the absence of other functional groups that would give strong absorption. 3. Confirm the identity of an unknown through fingerprint region. IR Spectroscopy Strengths and Limitations ¾IR alone cannot determine a structure. ¾Some signals may be ambiguous. ¾The functional group is usually indicated. ¾The absence of a signal is definite proof that the functional group is absent. ¾Correspondence with a known sample’s IR spectrum confirms the identity of the compound.