Types of Reactions in Organic Chemistry Chemistry
... Types of Reactions in Organic Chemistry The mechanism of a reaction is the detailed step-by-step description of how the overall reaction occurs. A substitution reaction is a chemical reaction in which an atom or group of atoms in a molecule is replaced by another atom or group of atoms. A substitut ...
... Types of Reactions in Organic Chemistry The mechanism of a reaction is the detailed step-by-step description of how the overall reaction occurs. A substitution reaction is a chemical reaction in which an atom or group of atoms in a molecule is replaced by another atom or group of atoms. A substitut ...
types of organic reactions
... One product will be there in greater amounts than the other and is called the major product (the other is called the minor product). To decide which is the major product, Markovnikov’s rule is used: The hydrogen atom of the addition reagent goes to the carbon atom of the double bond, that is attache ...
... One product will be there in greater amounts than the other and is called the major product (the other is called the minor product). To decide which is the major product, Markovnikov’s rule is used: The hydrogen atom of the addition reagent goes to the carbon atom of the double bond, that is attache ...
Alcohol Worksheet Key
... groups at position 3 and 5 will have very little effect on the acidity of the phenol. ...
... groups at position 3 and 5 will have very little effect on the acidity of the phenol. ...
Subject:
... particle size and the presence of a catalyst) 2-Use an energy graph to determine the following for a forward and reverse catalyzed and uncatalyzed reaction - ∆H, activation energy, potential energy of the products and reactants 3- Understand the concept of chemical equilibrium and calculate equilibr ...
... particle size and the presence of a catalyst) 2-Use an energy graph to determine the following for a forward and reverse catalyzed and uncatalyzed reaction - ∆H, activation energy, potential energy of the products and reactants 3- Understand the concept of chemical equilibrium and calculate equilibr ...
Chemical Kinetics - Review
... The series of steps by which an overall chemical reaction takes place is called the ________________. ...
... The series of steps by which an overall chemical reaction takes place is called the ________________. ...
Chap Thirteen: Alcohols
... ii. Reduction of Esters and Carboxylic Acids with LiAlH4 only LEARNING OUTCOMES: Understand the formation of alcohols through use of organometallics in carboncarbon bond forming reactions with electrophiles Predict the stereochemistry and optical activity of a product from an understanding of it ...
... ii. Reduction of Esters and Carboxylic Acids with LiAlH4 only LEARNING OUTCOMES: Understand the formation of alcohols through use of organometallics in carboncarbon bond forming reactions with electrophiles Predict the stereochemistry and optical activity of a product from an understanding of it ...
Inorganic Reaction Mechanisms at the Molecular Level Oxford
... Oxidation catalysts are typically based on the highest oxidation states of a particular metal complex, e.g., OsVIIIO4, RuVIIIO4, MnVIIO4-, etc. This seems consistent since the strongest “oxidants” should most efficiently catalyze “oxidation” reactions. However, this reasoning only holds if the rate ...
... Oxidation catalysts are typically based on the highest oxidation states of a particular metal complex, e.g., OsVIIIO4, RuVIIIO4, MnVIIO4-, etc. This seems consistent since the strongest “oxidants” should most efficiently catalyze “oxidation” reactions. However, this reasoning only holds if the rate ...
What is an addition reaction
... In a condensation reaction, two organic molecules react together to produce one larger organic molecule and a molecule of water. For this type of reaction to occur, one of the molecules must have a hydroxyl group, and the other must have an active site with hydrogens, such as another hydroxyl group, ...
... In a condensation reaction, two organic molecules react together to produce one larger organic molecule and a molecule of water. For this type of reaction to occur, one of the molecules must have a hydroxyl group, and the other must have an active site with hydrogens, such as another hydroxyl group, ...
Redox Reactions
... 3) Addition of H-, H+ – hydride reagents. Hydride reactions are nucleophilic reactions that reduce polar compounds. They are typically followed by an aqueous or acidic work-up that provides the proton. Addition of H2 These reductions rely on transition metal catalysts to split a molecule of H2 and t ...
... 3) Addition of H-, H+ – hydride reagents. Hydride reactions are nucleophilic reactions that reduce polar compounds. They are typically followed by an aqueous or acidic work-up that provides the proton. Addition of H2 These reductions rely on transition metal catalysts to split a molecule of H2 and t ...
study note 3 33
... are, in some cases, addition reactions. Polymerization, in some cases, may also proceed via addition reactions. Hydrolysis is a reaction in which water is one of the reactants. A reaction in which water is one of the products. Esterification is an example of a condensation reaction. In certain cases ...
... are, in some cases, addition reactions. Polymerization, in some cases, may also proceed via addition reactions. Hydrolysis is a reaction in which water is one of the reactants. A reaction in which water is one of the products. Esterification is an example of a condensation reaction. In certain cases ...
Reactions of Hydrocarbons & their functional groups
... 3) ELIMINATION REACTION • atoms are removed form a molecule to form double bonds. • Reverse of addition • To recognize: One reactant breaks into two products ...
... 3) ELIMINATION REACTION • atoms are removed form a molecule to form double bonds. • Reverse of addition • To recognize: One reactant breaks into two products ...
Table
... Condensation Reaction Pathway to other compounds Ester+NaOH sodium salt of acid+ alcohol Hydrolysis; saponification Preparation Amines: RX+NH3 amine + HX RX+R2NH amine +HX Amide +H2Ocarboxyic acid + amine (hydrolysis reaction) Amides Carboxylic acid + amine amide + H2O (condensation reaction) ...
... Condensation Reaction Pathway to other compounds Ester+NaOH sodium salt of acid+ alcohol Hydrolysis; saponification Preparation Amines: RX+NH3 amine + HX RX+R2NH amine +HX Amide +H2Ocarboxyic acid + amine (hydrolysis reaction) Amides Carboxylic acid + amine amide + H2O (condensation reaction) ...
Properties of Hydrocarbons
... Where an atom or group of atoms is displaced by an atom or group of atoms CH4 + Br2 → CH3Br + HBr Non specific reaction. Can not control which hydrogen is substituted or how many are substituted CH3Br + Br2 → CH2Br2 + HBr ...
... Where an atom or group of atoms is displaced by an atom or group of atoms CH4 + Br2 → CH3Br + HBr Non specific reaction. Can not control which hydrogen is substituted or how many are substituted CH3Br + Br2 → CH2Br2 + HBr ...
organic quiz 2
... 17) In a reaction between hex-2-ene and hydrochloric acid (aqueous hydrogen chloride), which of the following will be the product(s)? a) 1-chlorohexane only b) 2-chlorohexane only c) 3-chlorohexane only d) both (b) and (c) 18) DNA is a natural polymer composed of a) glucose monomers b) nucleotide mo ...
... 17) In a reaction between hex-2-ene and hydrochloric acid (aqueous hydrogen chloride), which of the following will be the product(s)? a) 1-chlorohexane only b) 2-chlorohexane only c) 3-chlorohexane only d) both (b) and (c) 18) DNA is a natural polymer composed of a) glucose monomers b) nucleotide mo ...
III. ORGANIC CHEMISTRY Reactions
... substitution reactions occur one step at a time; therefore, two substitutions cannot (and do not) take place at the same time (ex. bromine is diatomic, meaning two atoms of bromine are available for a substitution; however, each bromine gets added to a separate hydrocarbon, yielding a HBr molecule i ...
... substitution reactions occur one step at a time; therefore, two substitutions cannot (and do not) take place at the same time (ex. bromine is diatomic, meaning two atoms of bromine are available for a substitution; however, each bromine gets added to a separate hydrocarbon, yielding a HBr molecule i ...
TYPES OF REACTIONS IN ORGANIC CHEMISTRY
... ~ Monomers are the small molecules from which the polymer is made. ~ Molecules of ethene link with each other to form polythene. ~ Molecules of propene link with each other polypropene ~ Crude oil, a source of alkenes, is the raw material for the manufacture of ...
... ~ Monomers are the small molecules from which the polymer is made. ~ Molecules of ethene link with each other to form polythene. ~ Molecules of propene link with each other polypropene ~ Crude oil, a source of alkenes, is the raw material for the manufacture of ...
Nucleophilic Substitution Reaction
... In analogy with substitution reaction,b-elimination reactions are divided into E1 (Elimination, unimolecular) and E2 (Elimination, bimolecular) reactions. (i) E2 reaction : The Bimolecular mechanism Most of the elimination reactions are successful only when carried out in the presence of a strong ba ...
... In analogy with substitution reaction,b-elimination reactions are divided into E1 (Elimination, unimolecular) and E2 (Elimination, bimolecular) reactions. (i) E2 reaction : The Bimolecular mechanism Most of the elimination reactions are successful only when carried out in the presence of a strong ba ...
Synthesis of a Family of Chiral Asymmetric Schiff - Blogs at H-SC
... Carbon-Carbon bond-forming reactions are essential synthetic methods for organic chemists. Condensation reactions of carbonyl compounds are an important class of such reactions. Chiral organometallic compounds have been shown in some cases to act as catalysts to give condensation products in high yi ...
... Carbon-Carbon bond-forming reactions are essential synthetic methods for organic chemists. Condensation reactions of carbonyl compounds are an important class of such reactions. Chiral organometallic compounds have been shown in some cases to act as catalysts to give condensation products in high yi ...
COUPLING REACTIONS IN ORGANIC SYNTHESIS
... The addition of dihydrogen to Vaska's complex and other transition metals is a reversible reaction. The hydrogen can be released again if the reaction moves to the left in a reductive elimination. That reversibility makes transition metal compounds useful for hydrogen storage. Hydrogen gas is volumi ...
... The addition of dihydrogen to Vaska's complex and other transition metals is a reversible reaction. The hydrogen can be released again if the reaction moves to the left in a reductive elimination. That reversibility makes transition metal compounds useful for hydrogen storage. Hydrogen gas is volumi ...
Chemical Equations Balancing Chemical Equations Try One…
... review writing, balancing and naming the reaction type. In a chemical reaction, only 2 things are conserved the number of atoms and the conserved... number of grams. an arrow is used to separate reactants (the starting substances) and the products (what is made), the arrow is the same as an “equ ...
... review writing, balancing and naming the reaction type. In a chemical reaction, only 2 things are conserved the number of atoms and the conserved... number of grams. an arrow is used to separate reactants (the starting substances) and the products (what is made), the arrow is the same as an “equ ...
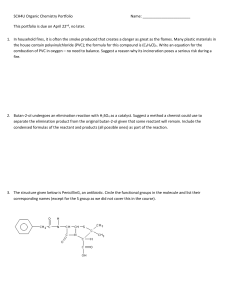
SCH4U Organic Chemistry Portfolio Name: This portfolio is due on
... 1. In household fires, it is often the smoke produced that creates a danger as great as the flames. Many plastic materials in the house contain polyvinylchloride (PVC); the formula for this compound is (C2H3Cl)n. Write an equation for the combustion of PVC in oxygen – no need to balance. Suggest a r ...
... 1. In household fires, it is often the smoke produced that creates a danger as great as the flames. Many plastic materials in the house contain polyvinylchloride (PVC); the formula for this compound is (C2H3Cl)n. Write an equation for the combustion of PVC in oxygen – no need to balance. Suggest a r ...
Organic Chemistry –Syllabus- one Semester Sackler faculty of
... double bond equivalent, alkyl group, Nomenclature (IUPAC rules), intermolecular forces( van der Waals force, Dipole–dipole interaction, Hydrogen bonds), Solubility, Conformations of alkanes(staggered-eclipsd) , Cycloalkanes, geometric isomers, The chair conformation of cyclohexane, Combustion of alk ...
... double bond equivalent, alkyl group, Nomenclature (IUPAC rules), intermolecular forces( van der Waals force, Dipole–dipole interaction, Hydrogen bonds), Solubility, Conformations of alkanes(staggered-eclipsd) , Cycloalkanes, geometric isomers, The chair conformation of cyclohexane, Combustion of alk ...
Mechanism
... has come up with a strange and unexpected product. Give a mechanism to explain his reaction. ...
... has come up with a strange and unexpected product. Give a mechanism to explain his reaction. ...
Ene reaction

The ene reaction (also known as the Alder-ene reaction) is a chemical reaction between an alkene with an allylic hydrogen (the ene) and a compound containing a multiple bond (the enophile), in order to form a new σ-bond with migration of the ene double bond and 1,5 hydrogen shift. The product is a substituted alkene with the double bond shifted to the allylic position.This transformation is a group transfer pericyclic reaction, and therefore, usually requires highly activated substrates and/or high temperatures. Nonetheless, the reaction is compatible with a wide variety of functional groups that can be appended to the ene and enophile moieties. Also,many useful Lewis acid-catalyzed ene reactions have been developed which can afford high yields and selectivities at significantly lower temperatures, making the ene reaction a useful C–C forming tool for the synthesis of complex molecules and natural products.