Microsoft Word
... diamines is obtained (<5%) yield and the conversion is not synthetically useful. Chapter 2 Section A: Synthesis of chiral guanidines and their application in stereoselective reactions: Guanidines are of considerable interest in biology and synthetic organic chemistry. Although there are many biolog ...
... diamines is obtained (<5%) yield and the conversion is not synthetically useful. Chapter 2 Section A: Synthesis of chiral guanidines and their application in stereoselective reactions: Guanidines are of considerable interest in biology and synthetic organic chemistry. Although there are many biolog ...
Dehydration notes-1
... 1. E1 – unimolecular transition state – formation of carbocation intermediate – Preferred for tertiary carbons ...
... 1. E1 – unimolecular transition state – formation of carbocation intermediate – Preferred for tertiary carbons ...
Workshop 5
... Consider the chlorination of methane. The initiation step involves the dissociation of Cl2 using either light or heat. Alternatively, tetramethyl lead ((CH3)4Pb) can be added to initiate this same reaction at a lower temperature. The Pb-C bond energy in (CH3)4Pb is 49 kcal/mol. a. Show the initiatio ...
... Consider the chlorination of methane. The initiation step involves the dissociation of Cl2 using either light or heat. Alternatively, tetramethyl lead ((CH3)4Pb) can be added to initiate this same reaction at a lower temperature. The Pb-C bond energy in (CH3)4Pb is 49 kcal/mol. a. Show the initiatio ...
3.8 ADDITION OF WATER TO AN ALKENE H or enzyme + H-O
... Our example just shows one molecule of monomer as a reactant, although in fact there are large numbers of them that will react with each other to form the polymer: The one sided arrows indicate that one electron goes to each C atom. This is in contrast to the previous reaction pathways where both el ...
... Our example just shows one molecule of monomer as a reactant, although in fact there are large numbers of them that will react with each other to form the polymer: The one sided arrows indicate that one electron goes to each C atom. This is in contrast to the previous reaction pathways where both el ...
DODH by Molybdenum Innovation Introduction DODH by Rhenium
... reductant and catalyst were carried out. To the right are shown the kinetic profiles for the standard experiment (green), less reductant (blue) and less catalyst (red), figure 5. This kinetic behaviour can be explained by a catalytic cycle driven by the reduction of Re(VII) to Re(V) by oxidation of ...
... reductant and catalyst were carried out. To the right are shown the kinetic profiles for the standard experiment (green), less reductant (blue) and less catalyst (red), figure 5. This kinetic behaviour can be explained by a catalytic cycle driven by the reduction of Re(VII) to Re(V) by oxidation of ...
lect7
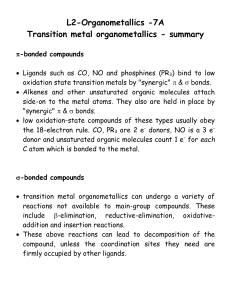
... the 18-electron rule. CO, PR3 are 2 e- donors, NO is a 3 edonor and unsaturated organic molecules count 1 e- for each C atom which is bonded to the metal. ...
... the 18-electron rule. CO, PR3 are 2 e- donors, NO is a 3 edonor and unsaturated organic molecules count 1 e- for each C atom which is bonded to the metal. ...
Chapter 7 Alkenes and Alkynes I
... removing sterically hindered hydrogens and generally only react with more accessible hydrogens (e.g. primary hydrogens) ...
... removing sterically hindered hydrogens and generally only react with more accessible hydrogens (e.g. primary hydrogens) ...
18 Important and sometimes forgotten) organic transformations
... •Phosphines can also be used •DMAP and DBU are better in some cases ...
... •Phosphines can also be used •DMAP and DBU are better in some cases ...
2015 CH 420 Take Home Quiz 3 March 24
... an arrow to the more nucleophilic nitrogen atom in the hydrazine substrate. In addition, circle the most electrophilic carbonyl in the 1,3-diketone substrate. ...
... an arrow to the more nucleophilic nitrogen atom in the hydrazine substrate. In addition, circle the most electrophilic carbonyl in the 1,3-diketone substrate. ...
Lecture 21 Enzyme mechanisms
... the presence of charge group near the active site, the pK’s of amino acid side chains in proteins may vary by several units from their nominal values. Distributions of charge near the active sites of enzymes are arranged in such a way that it can stabilize the transition states of the catalyzed reac ...
... the presence of charge group near the active site, the pK’s of amino acid side chains in proteins may vary by several units from their nominal values. Distributions of charge near the active sites of enzymes are arranged in such a way that it can stabilize the transition states of the catalyzed reac ...
Mechanistic Assignment
... Give an example of a molecule containing a nitrile in which it would be better to use an acidcatalyzed hydrolysis (instead of a base-catalyzed hydrolysis). Briefly explain your choice. ...
... Give an example of a molecule containing a nitrile in which it would be better to use an acidcatalyzed hydrolysis (instead of a base-catalyzed hydrolysis). Briefly explain your choice. ...
Rapid, Controlled Assembly of Polyenes for Studying Pericyclic
... Rapid, Controlled Assembly of Polyenes for Studying Pericyclic Reaction Cascades David A. Vosburg, Department of Chemistry, Harvey Mudd College Pericyclic reactions are among the most powerful transformations in organic chemistry, and they are even more impressive when they occur in tandem. Outstand ...
... Rapid, Controlled Assembly of Polyenes for Studying Pericyclic Reaction Cascades David A. Vosburg, Department of Chemistry, Harvey Mudd College Pericyclic reactions are among the most powerful transformations in organic chemistry, and they are even more impressive when they occur in tandem. Outstand ...
Chemistry 218, Winter 2007 Exam 2 Name: 1.
... ester protecting group is to treat it with sodium methoxide (followed by an acidic ...
... ester protecting group is to treat it with sodium methoxide (followed by an acidic ...
Document
... Cyclopropanes can be readily prepared by the addition of a carbene to the double bond of an alkene. A carbene has the general structure, R2C:, in which the central carbon is surrounded by six electrons (sextet), and is thus electron deficient. The electron-deficient carbene readily adds to an electr ...
... Cyclopropanes can be readily prepared by the addition of a carbene to the double bond of an alkene. A carbene has the general structure, R2C:, in which the central carbon is surrounded by six electrons (sextet), and is thus electron deficient. The electron-deficient carbene readily adds to an electr ...
Chapter 9. Addition Reactions of Alkenes
... The reaction below, which provides compound M as its major product, appears to defy the principles that we discussed in class. Draw the structures of the intermediate carbocations that form in this reaction, then clearly but briefly explain why M, and not L, is the major product of this reaction. Hi ...
... The reaction below, which provides compound M as its major product, appears to defy the principles that we discussed in class. Draw the structures of the intermediate carbocations that form in this reaction, then clearly but briefly explain why M, and not L, is the major product of this reaction. Hi ...
Chapter 13 - WebAssign
... reaction shown in Figure 13.26. In this reaction, an ester reacts with water to produce a carboxylic acid and an alcohol. Write a step-wise mechanism for the following reaction: O + H2O O ...
... reaction shown in Figure 13.26. In this reaction, an ester reacts with water to produce a carboxylic acid and an alcohol. Write a step-wise mechanism for the following reaction: O + H2O O ...
11 - DR CLEM KUEK
... • polymerises to produce polyethene. Since ethene is a small, non-polar molecule, the only attractive forces between its molecules are dispersion forces and ethene therefore has a very low boiling temperature: –104°C. With its double covalent bond, Ethene reacts more readily, and with more chemicals ...
... • polymerises to produce polyethene. Since ethene is a small, non-polar molecule, the only attractive forces between its molecules are dispersion forces and ethene therefore has a very low boiling temperature: –104°C. With its double covalent bond, Ethene reacts more readily, and with more chemicals ...
Exam 2 SOLUTION
... 1. Predict the product, or give the starting material for the following reactions: [12] ...
... 1. Predict the product, or give the starting material for the following reactions: [12] ...
Addition reactions
... bond the H (the E+) adds to the C with the most hydrogens & the X (the Nu:) adds to the most substituted carbon. - Vladimir V. Markovnikov, Russian, 1870 Substrate is an alkene & acts as the Nu: ...
... bond the H (the E+) adds to the C with the most hydrogens & the X (the Nu:) adds to the most substituted carbon. - Vladimir V. Markovnikov, Russian, 1870 Substrate is an alkene & acts as the Nu: ...
Assignment 4 Task 1a
... have been assigned to a new case and are working as part of a team to solve the case. Working in the laboratory you will need to have a good understanding of the conventions adopted to ensure that all chemical compounds have unambiguous names. You also need to understand how a combination of element ...
... have been assigned to a new case and are working as part of a team to solve the case. Working in the laboratory you will need to have a good understanding of the conventions adopted to ensure that all chemical compounds have unambiguous names. You also need to understand how a combination of element ...
Name: Chem 22 Final exam Spring `00 What product is formed when
... e) addtion of a hydride ion and a proton more or less at the same time 18. Which of the following describes “reductive amination?” a) an aldehyde or a ketone + a tertiary amine + H2/zeolite b) an aldehyde or a ketone + ammonia or a primary or a secondary amine + ...
... e) addtion of a hydride ion and a proton more or less at the same time 18. Which of the following describes “reductive amination?” a) an aldehyde or a ketone + a tertiary amine + H2/zeolite b) an aldehyde or a ketone + ammonia or a primary or a secondary amine + ...
Document
... 6) The McLafferty rearrangement In this rearrangement, an electron is donated through space; the other electron to form the bond comes from a bond to this atom. The initial cleavage does not cause fragmentation, only rearrangement and transfer of the radical site. This new radical site then initiate ...
... 6) The McLafferty rearrangement In this rearrangement, an electron is donated through space; the other electron to form the bond comes from a bond to this atom. The initial cleavage does not cause fragmentation, only rearrangement and transfer of the radical site. This new radical site then initiate ...
1 - Wikispaces
... (b) Propanoic acid can be prepared from propanal, CH3CH2CHO. State the reagents for this conversions. Reagents……………………………………………………………………………………………….. ...
... (b) Propanoic acid can be prepared from propanal, CH3CH2CHO. State the reagents for this conversions. Reagents……………………………………………………………………………………………….. ...
Lecture 8
... Enthalpy change does depend on conditions of temperature, pressure and concentration of the initial and final states, and it is important to specify these. Standard states are defined as pure substances at standard pressure (1bar), and temperature(298K). ...
... Enthalpy change does depend on conditions of temperature, pressure and concentration of the initial and final states, and it is important to specify these. Standard states are defined as pure substances at standard pressure (1bar), and temperature(298K). ...
organic lab questions
... Write descriptive organic equations for all reactions. If no reaction occurred, write N.R. for the products of the reaction. Under each alcohol, write the common name of the alcohol. Under each carboxylic acid write the I.U.P.A.C. name of the carboxylic acid. Finally, write the name of the ester und ...
... Write descriptive organic equations for all reactions. If no reaction occurred, write N.R. for the products of the reaction. Under each alcohol, write the common name of the alcohol. Under each carboxylic acid write the I.U.P.A.C. name of the carboxylic acid. Finally, write the name of the ester und ...
Ene reaction

The ene reaction (also known as the Alder-ene reaction) is a chemical reaction between an alkene with an allylic hydrogen (the ene) and a compound containing a multiple bond (the enophile), in order to form a new σ-bond with migration of the ene double bond and 1,5 hydrogen shift. The product is a substituted alkene with the double bond shifted to the allylic position.This transformation is a group transfer pericyclic reaction, and therefore, usually requires highly activated substrates and/or high temperatures. Nonetheless, the reaction is compatible with a wide variety of functional groups that can be appended to the ene and enophile moieties. Also,many useful Lewis acid-catalyzed ene reactions have been developed which can afford high yields and selectivities at significantly lower temperatures, making the ene reaction a useful C–C forming tool for the synthesis of complex molecules and natural products.