Calculating Percent Yield
... (Friedel-Crafts Alkylation) that incorporates the effect of substitution of the aromatic ring into the experiment. Students will prepare the product the first week of the experiment. During the second week, students will analyze the products by TLC analysis and melting point determination. In additi ...
... (Friedel-Crafts Alkylation) that incorporates the effect of substitution of the aromatic ring into the experiment. Students will prepare the product the first week of the experiment. During the second week, students will analyze the products by TLC analysis and melting point determination. In additi ...
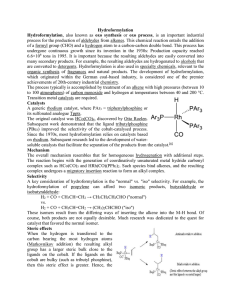
Hydroformylation Hydroformylation, also known as oxo synthesis or
... Hydroformylation Hydroformylation, also known as oxo synthesis or oxo process, is an important industrial process for the production of aldehydes from alkenes. This chemical reaction entails the addition of a formyl group (CHO) and a hydrogen atom to a carbon-carbon double bond. This process has und ...
... Hydroformylation Hydroformylation, also known as oxo synthesis or oxo process, is an important industrial process for the production of aldehydes from alkenes. This chemical reaction entails the addition of a formyl group (CHO) and a hydrogen atom to a carbon-carbon double bond. This process has und ...
Arenes test - A-Level Chemistry
... Use your answer to (i) to explain why bromine reacts much more readily with cyclohexene than it does with benzene. ...
... Use your answer to (i) to explain why bromine reacts much more readily with cyclohexene than it does with benzene. ...
Chemical Reactions - thsicp-23
... and form a compound. (Sometimes these are called combination or addition reactions.) reactant + reactant 1 product Basically: A + B AB ...
... and form a compound. (Sometimes these are called combination or addition reactions.) reactant + reactant 1 product Basically: A + B AB ...
2.10 Organic synthesis – Oxidation of alcohols
... • The reaction will proceed at a constant temperature (i.e. the solvent's boiling point.). •Any vapours given off are cooled back to liquid, and fall back into the reaction vessel • Useful for performing chemical reactions under controlled conditions that require substantial time for completion. ...
... • The reaction will proceed at a constant temperature (i.e. the solvent's boiling point.). •Any vapours given off are cooled back to liquid, and fall back into the reaction vessel • Useful for performing chemical reactions under controlled conditions that require substantial time for completion. ...
Eliminations
... SN1/SN2/E2/E1 summary/comparison: (1) Primary alkyl halides will prefer SN2 unless a strong hindered base is used in which case E2 will be favored. For example, t-‐butoxide is a sterically hindered base. ...
... SN1/SN2/E2/E1 summary/comparison: (1) Primary alkyl halides will prefer SN2 unless a strong hindered base is used in which case E2 will be favored. For example, t-‐butoxide is a sterically hindered base. ...
handout alkenes from alcohols
... hydroxyl group in R-OH is a poor-leaving group because it would have to leave as a hydroxide ion (HO-). Therefore, an acid is used to protonate the alcohol (step 1) and form R-OH2+ (see Figure 2). Thus, water (a much better leaving group) is the leaving group in this reaction (step 2) and the produc ...
... hydroxyl group in R-OH is a poor-leaving group because it would have to leave as a hydroxide ion (HO-). Therefore, an acid is used to protonate the alcohol (step 1) and form R-OH2+ (see Figure 2). Thus, water (a much better leaving group) is the leaving group in this reaction (step 2) and the produc ...
syllabus chemical science - SLET-NE
... due to ambiguity and vagueness in language. The candidates are also supposed to have a general acquaintance with the nature of a concept, meaning and criteria of truth, and the source of knowledge. There will be 60 questions, out of which the candidates can attempt any 50. In the event of the candid ...
... due to ambiguity and vagueness in language. The candidates are also supposed to have a general acquaintance with the nature of a concept, meaning and criteria of truth, and the source of knowledge. There will be 60 questions, out of which the candidates can attempt any 50. In the event of the candid ...
IB Chemistry Brakke ECA - Topic 15 T15D12
... The absolute entropy values, S, at 238 K for N2(g), H2(g) and NH3(g) are 192, 131 and 193 J K ο ο respectively. Calculate ∆S for the reaction and explain the sign of ∆S . ...
... The absolute entropy values, S, at 238 K for N2(g), H2(g) and NH3(g) are 192, 131 and 193 J K ο ο respectively. Calculate ∆S for the reaction and explain the sign of ∆S . ...
Procedure Notes
... due to the fact that the reaction is in equilibrium and esters are not very stable. The addition of water and heat from perspiration can cause the reaction to favor the reactants. Carboxylic acids tend to be associated with a foul smelling odor that one would not want to wear on their body. • Esters ...
... due to the fact that the reaction is in equilibrium and esters are not very stable. The addition of water and heat from perspiration can cause the reaction to favor the reactants. Carboxylic acids tend to be associated with a foul smelling odor that one would not want to wear on their body. • Esters ...
Addition Reactions
... acid-catalyzed hydration of an alkene is regioselective; hydrogen adds preferentially to the sp2 carbon with less # of hydrogens. ...
... acid-catalyzed hydration of an alkene is regioselective; hydrogen adds preferentially to the sp2 carbon with less # of hydrogens. ...
Chapter 13 - WebAssign
... What is the difference between saturated and unsaturated hydrocarbons? Alkanes contain only single bonds and are saturated hydrocarbons because they cannot react with hydrogen to produce more C-H bonds. However, multiple (double or triple) bonds are unsaturated because an H2 molecule can add across ...
... What is the difference between saturated and unsaturated hydrocarbons? Alkanes contain only single bonds and are saturated hydrocarbons because they cannot react with hydrogen to produce more C-H bonds. However, multiple (double or triple) bonds are unsaturated because an H2 molecule can add across ...
Chem 30BL_Lecture 2_.. - UCLA Chemistry and Biochemistry
... enthalpy (DH=23.9 kJ, ) nor the entropy (DS=84.91 J, ) changes much in the reaction and they also display opposing trends. Thus, the equilibrium constant is Keq=1.8 at 25 oC and Keq=8 at 80 oC, which are both low. ...
... enthalpy (DH=23.9 kJ, ) nor the entropy (DS=84.91 J, ) changes much in the reaction and they also display opposing trends. Thus, the equilibrium constant is Keq=1.8 at 25 oC and Keq=8 at 80 oC, which are both low. ...
Notes on Substitutions and Eliminations
... elimination. This is often an organic halide, but it is not limited to those. It is any organic molecule with a group that can be removed; a molecule that has an sp3-hybridized carbon attached to something more electronegative, creating a polar bond. You will need to recognize whether that carbon is ...
... elimination. This is often an organic halide, but it is not limited to those. It is any organic molecule with a group that can be removed; a molecule that has an sp3-hybridized carbon attached to something more electronegative, creating a polar bond. You will need to recognize whether that carbon is ...
Microsoft Word
... alcohol 33, which was then converted to the ketone-1,3-dithio acetal 34 (Scheme8). However, further oxidation of the dithio acetal 34 yielded the acid 35 in low yields and hence an alterate synthetic route was adopted. (Scheme-9) Figure In the scheme-9, allylic secondary alcohol 38, was prepared by ...
... alcohol 33, which was then converted to the ketone-1,3-dithio acetal 34 (Scheme8). However, further oxidation of the dithio acetal 34 yielded the acid 35 in low yields and hence an alterate synthetic route was adopted. (Scheme-9) Figure In the scheme-9, allylic secondary alcohol 38, was prepared by ...
Chem 30BL * Lecture 2 - UCLA Chemistry and Biochemistry
... enthalpy (DH=23.9 kJ, ) nor the entropy (DS=84.91 J, ) changes much in the reaction and they also display opposing trends. Thus, the equilibrium constant is Keq=1.8 at 25 oC and Keq=8 at 80 oC, which are both low. ...
... enthalpy (DH=23.9 kJ, ) nor the entropy (DS=84.91 J, ) changes much in the reaction and they also display opposing trends. Thus, the equilibrium constant is Keq=1.8 at 25 oC and Keq=8 at 80 oC, which are both low. ...
Review sheet - Paws.wcu.edu.
... can add e- density by resonance (-OH, -OR, -NR2, -Ph ) Deactivators: remove electron density from aromatic ring, reduce rate of EAS, direct meta can remove e- density by induction (-CF3, -N+R3, -SO3H, -NO2 ) can remove e- density by resonance (-NO2, -CN, -C(=O)-R carbonyl) Halogens: deactivators th ...
... can add e- density by resonance (-OH, -OR, -NR2, -Ph ) Deactivators: remove electron density from aromatic ring, reduce rate of EAS, direct meta can remove e- density by induction (-CF3, -N+R3, -SO3H, -NO2 ) can remove e- density by resonance (-NO2, -CN, -C(=O)-R carbonyl) Halogens: deactivators th ...
슬라이드 1
... Mixed copper-magnesium reagents analogous to the lithium cuprates can be prepared. These compounds are often called Normant reagents. The reagents undergo addition to terminal alkynes to generate alkenylcopper reagents. The addition is stereospecifically syn. ...
... Mixed copper-magnesium reagents analogous to the lithium cuprates can be prepared. These compounds are often called Normant reagents. The reagents undergo addition to terminal alkynes to generate alkenylcopper reagents. The addition is stereospecifically syn. ...
LOYOLA COLLEGE (AUTONOMOUS), CHENNAI – 600 034
... 12. Draw correlation diagram for the electrocyclization of 1,3,5-hexatriene for the dis-rotatory approach. State whether the reaction is feasible by thermal or photochemical means. 13. Predict the products in the following pericyclic reactions and explain the mechanism with ...
... 12. Draw correlation diagram for the electrocyclization of 1,3,5-hexatriene for the dis-rotatory approach. State whether the reaction is feasible by thermal or photochemical means. 13. Predict the products in the following pericyclic reactions and explain the mechanism with ...
CLASS-X SC (Chemical Reactions and Equations)
... 1. Copper displaces which of the following metals from its salt solution: (a) ZnSO4 (b) FeSO4 (c) AgNO3 (d) NiSO4 2. In an electrolytic cell where electrolysis is carried, anode has: (a) Positive change (b) Negative charge (c) Connected to negative terminal of the battery (d) None of these is correc ...
... 1. Copper displaces which of the following metals from its salt solution: (a) ZnSO4 (b) FeSO4 (c) AgNO3 (d) NiSO4 2. In an electrolytic cell where electrolysis is carried, anode has: (a) Positive change (b) Negative charge (c) Connected to negative terminal of the battery (d) None of these is correc ...
esters - wellswaysciences
... to form an ester and water is an equilibrium reaction the back reaction of this is the hydrolysis of an ester to reform the acid and the alcohol. • The reaction uses an aqueous (dilute) acid catalyst and is refluxed as before. • As before the reaction does not go to completion so all reactants and p ...
... to form an ester and water is an equilibrium reaction the back reaction of this is the hydrolysis of an ester to reform the acid and the alcohol. • The reaction uses an aqueous (dilute) acid catalyst and is refluxed as before. • As before the reaction does not go to completion so all reactants and p ...
DEHYDRATION - ALKENE TEST EXERCISES
... DEHYDRATION - ALKENE TEST EXERCISES 1. Give a detailed mechanism for the acid-catalyzed dehydration of cyclohexanol to cyclohexene. ...
... DEHYDRATION - ALKENE TEST EXERCISES 1. Give a detailed mechanism for the acid-catalyzed dehydration of cyclohexanol to cyclohexene. ...
Organic Dyes as Photoredox Catalysts
... amination of electron rich arenes with nitrogen heterocyclic nucleophiles.5 The value of this method in drug design and natural product synthesis was demonstrated in the late stage derivatization of biologically active molecules. Anilines were synthesized in a similar fashion using ammonium carbamat ...
... amination of electron rich arenes with nitrogen heterocyclic nucleophiles.5 The value of this method in drug design and natural product synthesis was demonstrated in the late stage derivatization of biologically active molecules. Anilines were synthesized in a similar fashion using ammonium carbamat ...
Ene reaction

The ene reaction (also known as the Alder-ene reaction) is a chemical reaction between an alkene with an allylic hydrogen (the ene) and a compound containing a multiple bond (the enophile), in order to form a new σ-bond with migration of the ene double bond and 1,5 hydrogen shift. The product is a substituted alkene with the double bond shifted to the allylic position.This transformation is a group transfer pericyclic reaction, and therefore, usually requires highly activated substrates and/or high temperatures. Nonetheless, the reaction is compatible with a wide variety of functional groups that can be appended to the ene and enophile moieties. Also,many useful Lewis acid-catalyzed ene reactions have been developed which can afford high yields and selectivities at significantly lower temperatures, making the ene reaction a useful C–C forming tool for the synthesis of complex molecules and natural products.