C h e m g u id e –... ESTERS: PREPARATION
... Explain what this equation means. b) Name the catalyst that is normally used for this reaction. c) If you were doing this reaction on a test tube scale, you would heat the mixture of carboxylic acid and alcohol with a few drops of the catalyst in a test tube stood in a hot water bath for a few minut ...
... Explain what this equation means. b) Name the catalyst that is normally used for this reaction. c) If you were doing this reaction on a test tube scale, you would heat the mixture of carboxylic acid and alcohol with a few drops of the catalyst in a test tube stood in a hot water bath for a few minut ...
effective: september 2003
... potential energy diagrams. Relative stabilities of cycloalkanes and ring strain. Banana bonds in cyclopropane, conformations of cylobutane and cyclohexane. Chair and boat forms of cyclohexane. Conformational analysis of substituted cyclohexa nes. Reduction of alkyl halides and lithium diakylcuprates ...
... potential energy diagrams. Relative stabilities of cycloalkanes and ring strain. Banana bonds in cyclopropane, conformations of cylobutane and cyclohexane. Chair and boat forms of cyclohexane. Conformational analysis of substituted cyclohexa nes. Reduction of alkyl halides and lithium diakylcuprates ...
18 - cloudfront.net
... With a lower activation-energy barrier, more reactants have the energy to form products within a given time. For instance, the rate of the combination reaction of hydrogen and oxygen at room temperature in negligible, but with a trace of finely divided platinum (Pt) as a catalyst, the reaction is ra ...
... With a lower activation-energy barrier, more reactants have the energy to form products within a given time. For instance, the rate of the combination reaction of hydrogen and oxygen at room temperature in negligible, but with a trace of finely divided platinum (Pt) as a catalyst, the reaction is ra ...
Lesson 4 halogenoalkanes
... • A bond begins to form between the oxygen and carbon atoms, at the SAME time the carbon-bromine bond weakens. • A transition state will form with a ½ O-C bond and ½ C-Br bond, only IF there was enough energy in the collision. • The O-C bond forms completely, the C-Br bond breaks completely NB - If ...
... • A bond begins to form between the oxygen and carbon atoms, at the SAME time the carbon-bromine bond weakens. • A transition state will form with a ½ O-C bond and ½ C-Br bond, only IF there was enough energy in the collision. • The O-C bond forms completely, the C-Br bond breaks completely NB - If ...
Types of Chemical Reactions Name_________________________
... opportunity to learn about the different types of chemical reactions. The website address for this assignment is www.ric.edu/ptiskus/reactions. On the website you will find a brief description of the main types of chemical reactions. There are several representative reactions listed for each main ty ...
... opportunity to learn about the different types of chemical reactions. The website address for this assignment is www.ric.edu/ptiskus/reactions. On the website you will find a brief description of the main types of chemical reactions. There are several representative reactions listed for each main ty ...
Determination of the reaction order Determination of the reaction
... Elementary reactions & complex reactions Chemical reactions usually do not occur as they are written in chemical equations, which represent their summarical stoichiometry only. Majority of reactions are – from the point of view of their kinetics – complex reactions. It means that their occur is sev ...
... Elementary reactions & complex reactions Chemical reactions usually do not occur as they are written in chemical equations, which represent their summarical stoichiometry only. Majority of reactions are – from the point of view of their kinetics – complex reactions. It means that their occur is sev ...
Chem 231 Exam #3 Study Guide
... Know the order of substrate reactivities for the different reactions Know how to predict nucleophilicity (two rules) and the relative order of nucleophiles in protic solvent Be able to predict a good versus bad leaving group Know how solvents effect SN1 versus SN2 reactions Know how to name alkenes ...
... Know the order of substrate reactivities for the different reactions Know how to predict nucleophilicity (two rules) and the relative order of nucleophiles in protic solvent Be able to predict a good versus bad leaving group Know how solvents effect SN1 versus SN2 reactions Know how to name alkenes ...
Enantioselective one-pot synthesis of dihydroquinolones via BINOL
... isolated products. c Enantioselectivities determined by chiral HPLC; diastereoselectivities determined by examination of crude 1H NMR spectra. In general, previous synthetic efforts towards the synthesis of dihydroquinolones have focused primarily on products with aromatic substituents, and generall ...
... isolated products. c Enantioselectivities determined by chiral HPLC; diastereoselectivities determined by examination of crude 1H NMR spectra. In general, previous synthetic efforts towards the synthesis of dihydroquinolones have focused primarily on products with aromatic substituents, and generall ...
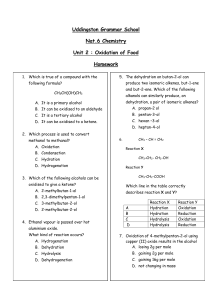
2d Oxidation of Food Homework
... Borane (BH3) is used to synthesis alcohols from alkenes. The reaction occurs in two stages ...
... Borane (BH3) is used to synthesis alcohols from alkenes. The reaction occurs in two stages ...
Exam 3 Review
... Describe hydrogen bonding in alcohols, and compare alcohol polarity to ether polarity. What are the acid/base properties of alcohols? Rank these compounds in order of acidity. How are Grignard reagents prepared? Describe their bond polarity. How do organolithiums react? Use the Williamson ether synt ...
... Describe hydrogen bonding in alcohols, and compare alcohol polarity to ether polarity. What are the acid/base properties of alcohols? Rank these compounds in order of acidity. How are Grignard reagents prepared? Describe their bond polarity. How do organolithiums react? Use the Williamson ether synt ...
Chapter 6: Alkynes, reactions of alkynes, and multistep synthesis
... c. easly get aldehyde 5. Hydrogenation a. usually can’t stop at alkene b. can stop only if using “poison” catalyst (Lindlar catalyst) c. get cis alkene for syn addition with Lindlar d. to get trans, use Na or Li in liquid ammonia (-78ºC) e. this is radical addition ...
... c. easly get aldehyde 5. Hydrogenation a. usually can’t stop at alkene b. can stop only if using “poison” catalyst (Lindlar catalyst) c. get cis alkene for syn addition with Lindlar d. to get trans, use Na or Li in liquid ammonia (-78ºC) e. this is radical addition ...
Organometallic Chemistry
... • The use of enantiopure allylic boranes in reactions with achiral aldehydes results not only in high diastereoselection, but also in high enantioselection. • Pure (Z)- and (E)- crotyldiisopino campheylboranes can be prepared at low temperature from (Z)- or (E)- crotylpotassium and B-methoxydiisopin ...
... • The use of enantiopure allylic boranes in reactions with achiral aldehydes results not only in high diastereoselection, but also in high enantioselection. • Pure (Z)- and (E)- crotyldiisopino campheylboranes can be prepared at low temperature from (Z)- or (E)- crotylpotassium and B-methoxydiisopin ...
Lecture 13a - UCLA Chemistry and Biochemistry
... (i.e., grinding, ultrasound, microwave), which often requires a phase transfer catalyst i.e., Aliquat 336® • Which method is preferential depends on the R-group on the aldehyde • R= NO2, F, Cl, Br, CF3: solution method preferred • R= alkyl (Me, Et, iso-Pr, tert. Bu), alkoxy (OMe, OEt), amino (NMe2, ...
... (i.e., grinding, ultrasound, microwave), which often requires a phase transfer catalyst i.e., Aliquat 336® • Which method is preferential depends on the R-group on the aldehyde • R= NO2, F, Cl, Br, CF3: solution method preferred • R= alkyl (Me, Et, iso-Pr, tert. Bu), alkoxy (OMe, OEt), amino (NMe2, ...
aldehyde ketone
... CH3-CH2-OH CH3-CHO + 2H+ + 2e CH3-CHO + H2O CH3COOH + 2H+ + 2e reduction; Cr2O72-(aq) + 14H+(aq) + 6e- 2Cr3+ (aq) +7H2O orange ...
... CH3-CH2-OH CH3-CHO + 2H+ + 2e CH3-CHO + H2O CH3COOH + 2H+ + 2e reduction; Cr2O72-(aq) + 14H+(aq) + 6e- 2Cr3+ (aq) +7H2O orange ...
1. Four of the structural isomers of C4H10O are alcohols. One of
... Calculate the maximum possible mass of butan-2-ol which could be obtained in the above experiment. ...
... Calculate the maximum possible mass of butan-2-ol which could be obtained in the above experiment. ...
Organic Reactions
... • Alkenes have pi bonds in which electrons are easily accessible because they aren’t trapped between two nuclei as sigma bonding electrons are. • Other functional groups have highly electronegative atoms like O, N or halogens ...
... • Alkenes have pi bonds in which electrons are easily accessible because they aren’t trapped between two nuclei as sigma bonding electrons are. • Other functional groups have highly electronegative atoms like O, N or halogens ...
Carbon-Carbon Bond Forming Reactions
... - must consider regioselectivity when using substituted benzenes directing effects: o/p or m (see handout) ...
... - must consider regioselectivity when using substituted benzenes directing effects: o/p or m (see handout) ...
ORGANIC REACTIONS IN A CLAY MICROENVIRONMENT
... Al-bentonite at 200~ it decomposed to give the same equilibrium mixture. The multiple equilibria which participate in this reaction sequence are shown in Scheme 3 which explains the formation of the observed products. If, however, alkenes are chosen which can only give a single carbocation intermedi ...
... Al-bentonite at 200~ it decomposed to give the same equilibrium mixture. The multiple equilibria which participate in this reaction sequence are shown in Scheme 3 which explains the formation of the observed products. If, however, alkenes are chosen which can only give a single carbocation intermedi ...
... 10. Arrange the following in terms of increasing acid strength and give reasons. Propionic acid , 2chloropropionic acid , 2 fluoropropionic acid. PART - B Answer any EIGHT questions (8 x 5 = 40) 11. Give a mechanism for the reaction of tert.butyl bromide with aqueous NaOH to form tert.butyl alcohol. ...
ORGANIC REACTIONS 14 APRIL 2015 Section A
... Fibres are produced by an elimination reaction known as condensation polymerisation. In this reaction two molecules with different functional groups react with each other, and a small molecule is removed (usually water) Polyactic acid (PLA) is produced from a monomer which comes the fermentation of ...
... Fibres are produced by an elimination reaction known as condensation polymerisation. In this reaction two molecules with different functional groups react with each other, and a small molecule is removed (usually water) Polyactic acid (PLA) is produced from a monomer which comes the fermentation of ...
a. Rank by acidity. The most acidic compound is 1, wh
... You may use any inorganic reagent you desire and any organic compound which contains four carbons or less. (15 points each) a. HC CH ...
... You may use any inorganic reagent you desire and any organic compound which contains four carbons or less. (15 points each) a. HC CH ...
Subject Description Form
... common spectroscopic techniques available for functional group identification. Illustration will be emphasized on reactions and compounds with structural interest or industrial importance. ...
... common spectroscopic techniques available for functional group identification. Illustration will be emphasized on reactions and compounds with structural interest or industrial importance. ...
handout alkenes from alcohols
... hydroxyl group in R-OH is a poor-leaving group because it would have to leave as a hydroxide ion (HO-). Therefore, an acid is used to protonate the alcohol (step 1) and form R-OH2+ (see Figure 2). Thus, water (a much better leaving group) is the leaving group in this reaction (step 2) and the produc ...
... hydroxyl group in R-OH is a poor-leaving group because it would have to leave as a hydroxide ion (HO-). Therefore, an acid is used to protonate the alcohol (step 1) and form R-OH2+ (see Figure 2). Thus, water (a much better leaving group) is the leaving group in this reaction (step 2) and the produc ...
Ene reaction

The ene reaction (also known as the Alder-ene reaction) is a chemical reaction between an alkene with an allylic hydrogen (the ene) and a compound containing a multiple bond (the enophile), in order to form a new σ-bond with migration of the ene double bond and 1,5 hydrogen shift. The product is a substituted alkene with the double bond shifted to the allylic position.This transformation is a group transfer pericyclic reaction, and therefore, usually requires highly activated substrates and/or high temperatures. Nonetheless, the reaction is compatible with a wide variety of functional groups that can be appended to the ene and enophile moieties. Also,many useful Lewis acid-catalyzed ene reactions have been developed which can afford high yields and selectivities at significantly lower temperatures, making the ene reaction a useful C–C forming tool for the synthesis of complex molecules and natural products.